The chemical formula for potassium cyanide is KCN. It appears as white amorphous lumps or a crystalline mass with a faint almond odor. This colorless crystalline salt, which resembles sugar in appearance, is highly soluble in water. The majority of KCN is employed in gold mining, organic synthesis, and electroplating. Jewelry for chemical gilding and buffing are examples of smaller applications. It is used in the extraction of gold and silver, chemical analysis, the production of other chemicals, and as an insecticide.
Potassium cyanide is extremely hazardous to one’s health. It emits hydrogen cyanide gas, a highly toxic chemical asphyxiant that disrupts the body’s ability to use oxygen. Due to hydrolysis, the moist solid emits trace amounts of hydrogen cyanide, which has a bitter almond aroma. However, not everyone can smell this; it is a genetic trait. Potassium cyanide has been described as having an acrid, bitter taste with a burning sensation similar to lye. Potassium cyanide poisoning can be lethal in a matter of minutes.
Properties
- Molecular Weight: 65.12 g/mol
- Density: 1.52 g/cm3
- Melting Point: 634.5 °C
- Boiling point: 1,625 °C
Production
Potassium cyanide is a cyanide salt that contains the same amount of potassium cations and cyanide anions as sodium cyanide. KCN is created by reacting hydrogen cyanide with an aqueous potassium hydroxide solution, then evaporating the solution in a vacuum:
HCN + KOH → KCN + H2O
Potassium cyanide solution is a colorless, clear aqueous solution with a faint bitter almond odor. Every year, approximately 50,000 tons of potassium cyanide are produced.

Historical production
Before 1900 and the invention of the Castner process, potassium cyanide was the most important source of alkali metal cyanides. In this historical process, potassium cyanide was produced by decomposing potassium ferrocyanide:
K4[Fe(CN)6] → 4 KCN + FeC2 + N2
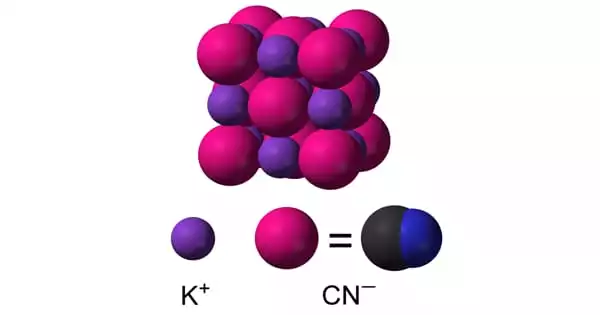
Structure
KCN is dissociated in aqueous solution into hydrated potassium (K+) ions and cyanide (CN) ions. The most common form of solid KCN, which is stable at room temperature and pressure, has the same cubic crystal structure as sodium chloride, with each potassium ion surrounded by six cyanide ions, and vice versa. Despite being diatomic and thus less symmetric than chloride, cyanide ions rotate so quickly that their time-averaged shape is spherical. This free rotation is hampered at low temperatures and high pressure, resulting in a less symmetric crystal structure with cyanide ions arranged in sheets.
Applications
KCN and sodium cyanide (NaCN) are widely used in organic synthesis, particularly in the von Richter reaction, to prepare nitriles and carboxylic acids.
In the wet plate collodion process, KCN is used as a photographic fixer. The KCN dissolves silver in areas where the developer has not rendered it insoluble. This reveals and stabilizes the image, rendering it no longer light sensitive. Although many modern wet plate photographers prefer less toxic fixers, such as sodium thiosulfate, KCN is still used.
Some common uses of KCN –
- It is used in the extraction of silver and gold from ores.
- It is used in the electroplating process.
- It used as a reagent in analytical chemistry.
- It is widely used in the preparation of carboxylic acids and nitriles.
- It is used in the wet plate collodion process as a photographic fixer.
- It is used in gold mining.
- It is used in warehouses.
Health Hazards
Potassium cyanide is hazardous to one’s health. If someone inhales this element, they may experience severe health problems, which may even result in death. A person may also experience headaches, vomiting, dizziness, and seizures, all of which can be dangerous to one’s health.