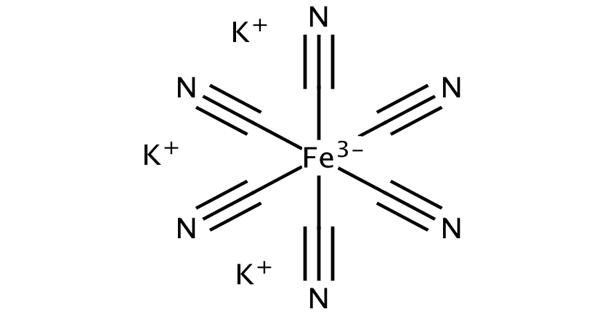
Potassium ferricyanide is the chemical compound with the formula K3[Fe(CN)6]. This chemical compound exists as a bright red salt under standard conditions for temperature and pressure (usually abbreviated to STP). This bright red salt contains the octahedrally coordinated [Fe(CN)6]3- ion. It is soluble in water and its solution shows some green-yellow fluorescence. It can be noted that the coordination structure of the ferricyanide anion is octahedral. It was discovered in 1822 by Leopold Gmelin,
Properties
Under standard conditions for temperature and pressure (usually abbreviated to STP), potassium ferricyanide is known to exist in the form of deep red crystals. However, it is not uncommon for this coordination compound to exist in the form of pellets or a powder which is either dark red or orange.
- Molecular Weight: 329.24
- Appearance: Orange to red powder or crystals
- Melting Point: N/A
- Boiling Point: N/A
- Density: N/A
- Solubility in H2O: N/A
- Exact Mass: 328.844502
- Monoisotopic Mass: 328.844502

Preparation
Potassium ferricyanide is known to be soluble in water. Furthermore, aqueous solutions of this coordination compound in water are also known to exhibit certain levels of green or greenish-yellow fluorescence. It is manufactured by passing chlorine through a solution of potassium ferrocyanide. Potassium ferricyanide separates from the solution:
2 K4[Fe(CN)6] + Cl2 → 2 K3[Fe(CN)6] + 2 KCl
When the chlorine gas is passed through the potassium ferrocyanide solution, the red coloured potassium ferricyanide is formed. This compound goes on to separate itself from the potassium ferrocyanide solution. The chemical equation for this reaction is provided below.
2K4[Fe(CN)6] + Cl2 → 2KCl + K3[Fe(CN)6]
Therefore, it can be understood that two molar equivalents of potassium chloride are obtained for each molar equivalent of chlorine gas passed through the potassium ferrocyanide solution.
Structure
Potassium ferricyanide is known to have an extremely complicated polymeric structure (as is usually the case with most metal cyanides). Like other metal cyanides, solid potassium ferricyanide has a complicated polymeric structure. It is important to note that the ferricyanide anion, which is sometimes referred to as the hexacyanoferrate(III) anion, is known to have an octahedral coordination geometry. The polymer consists of octahedral [Fe(CN)6]3- centers crosslinked with K+ ions that are bound to the CN ligands. The K+—NCFe linkages break when the solid is dissolved in water.
Applications
The compound is also used to harden iron and steel, in electroplating, dyeing wool, as a laboratory reagent, and as a mild oxidizing agent in organic chemistry.
- One of the most notable applications is in the Cyanotype process, where it is employed in the printing processes that yield cyan-blue prints.
- This compound is also known to have various applications in the field of photography.
- This compound can also be of use during the hardening of iron and the hardening of steel.
- The process of electroplating is often done using this compound as one of the raw materials.
- It is also known to be used in the dyeing of wool.
Toxicity
Potassium ferricyanide is known to have relatively low toxicity. However, the primary hazard associated with this compound is that it is a mild irritant to the skin and the eyes. It is also important to note that the highly toxic gas hydrogen cyanide can be produced when this compound is placed under very strongly acidic conditions.
Information Source: