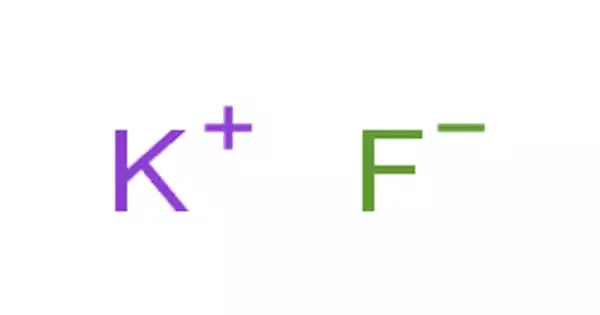Potassium fluoride is a chemical compound with the formula KF. It appears as white powder or crystals with a strong saline flavor. It is shipped as a solid or as an aqueous solution. It is highly irritating to the skin, eyes, and mucous membranes. The primary applications of potassium fluoride are in the metallurgical industry, such as soldering fluxes and tin plating. It is also widely used as a fluorinating agent in organic synthesis to replace other halide atoms with fluorine. It is toxic if consumed.
It is found in insecticides, solder flux, and glass etching. Following hydrogen fluoride, KF is the primary source of the fluoride ion for applications in manufacturing and chemistry. It is an alkali halide that occurs naturally as the rare mineral carobbiite. KF solutions will etch glass due to the formation of soluble fluorosilicates, but HF is more effective.
Properties
Potassium fluoride is the basic raw material used in the production of fluoride, and its chemical formula is KF. The atomic weight is 58.10. It is a white powder or colorless cubic crystal. It has a delectable flavor. The flavor is salty. 2.48 is the specific gravity. The melting point is 858°C. The boiling point is 1505°C. It dissolves in water. It was dissolved in ammonia and hydrofluoric acid. It is insoluble in acetone and ethanol.
- Molecular Weight: 58.09
- Appearance: White Powder
- Melting Point: 858° C (1,576° F)
- Boiling Point: 1,505° C (2,741° F)
- Density: 2.48 g/cm3
- Solubility in H2O: N/A
- Exact Mass: 57.962 Da

Preparation
Potassium fluoride is prepared by dissolving potassium carbonate in hydrofluoric acid. It is synthesized by reacting potassium carbonate with excess hydrofluoric acid and then evaporating the subsequent solution to form potassium bifluoride crystals. Evaporation of the solution forms crystals of potassium bifluoride. The bifluoride on heating yields potassium fluoride:
K2CO3 + 4HF → 2KHF2 + CO2↑ + H2O
KHF2 → KF + HF↑
Platinum or heat-resistant plastic containers are often used for these operations.
Potassium chloride converts to KF upon treatment with hydrogen fluoride. In this way, potassium fluoride is recyclable.
Crystalline properties
KF crystallizes in the cubic NaCl crystal structure. The lattice parameter at room temperature is 0.266 nm.
Applications in organic chemistry
KF can be used in organic chemistry to convert chlorocarbons to fluorocarbons via the Finkelstein (alkyl halides) and Halex reactions (aryl chlorides). Polar solvents such as dimethylformamide, ethylene glycol, and dimethyl sulfoxide are commonly used in such reactions. A combination of crown ether and bulky diols in acetonitrile solvent allows for more efficient fluorination of aliphatic halides.
In organic synthesis, anhydrous potassium fluoride is used as a catalyst for various reactions or to introduce fluorine into organic molecules. Fluoro compounds, for example, can be made by replacing labile chlorine atoms with fluorine atoms, as in the production of sodium fluoroacetate, a rat poison.
Safety considerations
KF, like other sources of the fluoride ion, F–, is poisonous, though lethal doses for humans approach gram levels. It is toxic when inhaled or ingested. It is extremely corrosive, and contact with the skin can result in severe burns.