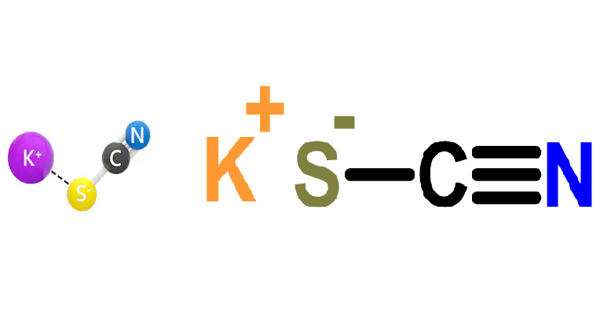Potassium thiocyanate is a potassium salt which is the monopotassium salt of thiocyanic acid. It is a chemical compound with the molecular formula KSCN. It is a colorless, crystalline, hygroscopic, water-soluble solid, KSCN, used chiefly in the manufacture of chemicals, dyes, and drugs. It is an important salt of the thiocyanate anion, one of the pseudohalides. The compound has a low melting point relative to most other inorganic salts.
Properties
- Form: solid
- pH: 5.3 – 8.7 at 97.2 g/l at 25 °C (77 °F)
- Melting point/range: 173 °C (343 °F) – lit.
- Boiling point: n/a
- Density: 1.890 g/cm3
- Water solubility: ca.97.2 g/l at 20 °C (68 °F)
- Molecular Weight: 97.18

Use in chemical synthesis
Potassium Thiocyanate solution can be used in titrations involving Silver Nitrate solutions. Aqueous KSCN reacts almost quantitatively with Pb(NO3)2 to give Pb(SCN)2, which has been used to convert acyl chlorides to isothiocyanates. Chloride, Bromide, and Iodide can be determined indirectly this way by adding excess Silver Nitrate solution to the sample containing the Halide, thus precipitating the Halide, and titrating the excess Silver Nitrate with Potassium Thiocyanate titrant using a Ferric Alum Indicator.
KSCN converts ethylene carbonate to ethylenesulfide. For this purpose, the KSCN is first melted under vacuum to remove water. In a related reaction, KSCN converts cyclohexene oxide to the corresponding episulfide.
C6H10O + KSCN → C6H10S + KOCN
KSCN is also the starting product for the synthesis of carbonyl sulfide.
Other uses
Thiocyanates can be used in different applications in the textile and fibre industry, in agriculture, in metal and steel industry and in construction.
Dilute aqueous KSCN is occasionally used for moderately realistic blood effects in film and theater. It can be painted onto a surface or kept as a colorless solution.
When in contact with ferric chloride solution (or other solutions containing Fe3+), the product of the reaction is a solution with a blood red color, due to the formation of the thiocyanatoiron complex ion.
Thus this chemical is often used to create the effect of ‘stigmata’. Because both solutions are colorless, they can be placed separately on each hand. When the hands are brought into contact, the solutions react and the effect looks remarkably like stigmata.
In a number of applications potassium thiocyanate can be used as an analytical reagent, in the synthesis of antibiotics and other pharmaceutical products, and in the electroplating of metals and surfaces.
Information Source: