Propionic acid (/proʊpiˈɒnɪk/), also known as propionate or acid propanoique, is a carboxylic acid with a chemical formula CH3CH2CO2H that occurs naturally. Carboxylic acids are compounds that contain a group of carboxylic acids with the formula -C(=O)OH. This is a transparent, colorless liquid boiling at 140° C (284 OF). It is flame-retardant; it has a pungent odor and is water- and alcohol soluble. The CH3CH2CO2− anion and propionic acid salts and esters are known as propionates or propanoates.
Propionic acid is a monocarboxylic acid of aliphatic origin. It is used in electroplating solutions for copper, perfumes, artificial flavors, pharmaceuticals, and propionates for processing. Propionic acid exists as a liquid, soluble (in water), and a compound which is weakly acidic (based on its pKa). It has been found all through most human tissues and has additionally been identified in many biofluids, including blood, spit, excrement, and pee. It was first portrayed in 1844 by Johann Gottlieb, who discovered it among the corruption results of sugar. Over the next few years, propionic acid was developed in many other forms by other chemists, none of whom knew they were creating the same product.
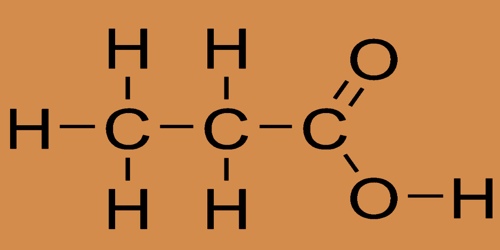
Full structural formula of Propionic Acid
Inside the cell, propionic corrosive is principally situated in the cytoplasm and mitochondria. It exists in all eukaryotes, running from yeast to people. This corrosive partakes in various enzymatic responses. In 1847, the French chemist Jean-Baptiste Dumas identified all the acids as the same compound, which he referred to as propionic acid, from the Greek terms ñanaff (prōtos), meaning first, and pioff (piōn), meaning fat, because it is the smallest H(CH2)nCOOH acid that exhibits the properties of other fatty acids, such as forming an oily layer when salted from water and providing a potassium salt in soap.
Propionic acid is really utilized in the production of propionates utilized as shape inhibitors and preservatives for grains and wood chips, in the assembling of organic product flavors and perfume bases, and as an esterifying specialist. It has physical properties middle between those of the littler carboxylic acids, formic and acidic acids, and the bigger unsaturated fats. It is miscible with water, yet can be taken out from the water by including salt. Similarly, as with acidic and formic acids, it comprises of hydrogen reinforced sets of atoms as both the fluid and the fume.
In particular, propionic acid can be biosynthesized from propinol adenylate; this is regulated by the short-chain family member enzyme acyl-CoA synthetase 3, mitochondrial. Moreover, through its association with the enzyme acetyl-coenzyme A synthetase 2-like, mitochondrial, this acid can be biosynthesized from propinol adenylate. It displays the final properties of carboxylic acids: it can form amide, ester, anhydride, and chloride derivatives.
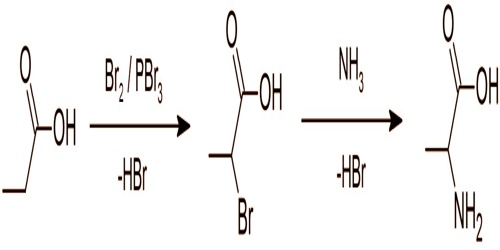
Preparation of alanine from Propionic Acid
In humans, propionic acid is active in the pathway of propanoate synthesis and the process of synthesis of vitamin K. It is also implicated in a few metabolic disorders, including the pathway to malonic aciduria, deficiency in malonyl-CoA decarboxylase, and methylmalonic aciduria due to cobalamin-related disorders. In industry, propionic acid is especially produced by the hydrocarboxylation of ethylene using nickel carbonyl because of the catalyst:
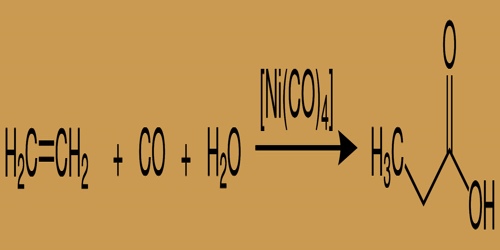
Industrial synthesis of Propionic Acid
Even propionic acid is produced through propionaldehyde aerobic oxidation. This reaction occurs rapidly at temperatures as mild as 40–50°C in the presence of cobalt or manganese salts (the most widely used manganese propionate):
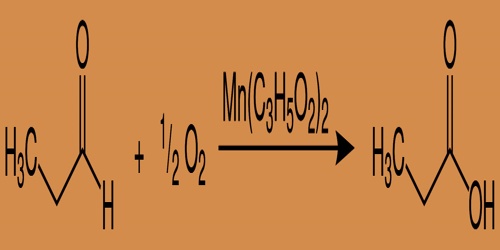
Industrial synthesis of propionic acid (Oxidation Process)
Propionic acid responds as a corrosive to kill bases in exothermic responses. Consumes when presented to warmth, fire, or oxidizers. At the point when warmed to decay transmits harsh smoke and disturbing vapor. Outside of the human body, propionic corrosive can be found in various food things, for example, winter squash, chicory, basic buckwheat, and green thread tea. This makes propionic acid a possible biomarker for certain food items to be eaten. Significant quantities of propionic acid were once generated as a by-product of manufacturing acetic acid. At present, BASF is the world’s largest producer of propionic acid, with a production capacity of about 150 kt/a.
Propionic acid is a poisonous and destructive fluid. Contact with the eyes can result in eye injury. Skin contact may cause consumes. Intense presentations to its fumes can cause eye redness, mellow to direct skin burns, and gentle hacking. This corrosive has been discovered to be related with a few maladies known as a peevish inside condition, Crohn’s sickness, and ulcerative colitis; propionic corrosive has additionally been connected to a few inalienable metabolic issues including celiac illness and propionic acidemia.
Propionic acid is a short-chain, saturated fatty acid composed of ethane bound to a carboxy group’s carbon. It’s also used for producing pesticides and pharmaceuticals in more advanced applications. The pure type of propionic acid is corrosive and is likely to cause burns to any contact region. Additionally, being a three-carbon atom takes care of into hepatic gluconeogenesis (that is, the production of glucose particles from more straightforward particles in the liver). It has a function as an antifungal medication. It is a short-chain unsaturated fat and soaked unsaturated fat. It is a form corrosive of a propionate.
Even if the propionic acid is solid, it is flammable. It should be kept away from heat and fire, in an airtight jar. Propionic acid is a popular intermediate product in biogas plants which is produced by fermentation with propionic acid bacteria. It requires the involvement of complex microbial communities to degrade in anaerobic environments (e.g., biogas plants).
Information Sources: