The demand for clean energy has never been greater, resulting in a global race to develop new technologies as alternatives to fossil fuels. Fuel cells are one of the most enticing of these green energy technologies. They use hydrogen as a fuel to generate electricity in an environmentally friendly manner and could power everything from long-haul trucks to major industrial processes. Researchers have discovered new dynamics that have the potential to speed up a sluggish part of the core chemical reaction in fuel cells.
The demand for clean energy has never been greater, resulting in a global race to develop new technologies as alternatives to fossil fuels. Fuel cells are one of the most enticing of these green energy technologies. They use hydrogen as a fuel to generate electricity in an environmentally friendly manner and could power everything from long-haul trucks to major industrial processes.
However, fuel cells are hampered by slow kinetics in a critical part of the chemical reaction, which limits efficiency. However, the University of Texas at Austin researchers have discovered new dynamics that could supercharge this reaction using iron-based single-atom catalysts.
Researchers have discovered new dynamics that could supercharge a sluggish part of the core chemical reaction in fuel cells.
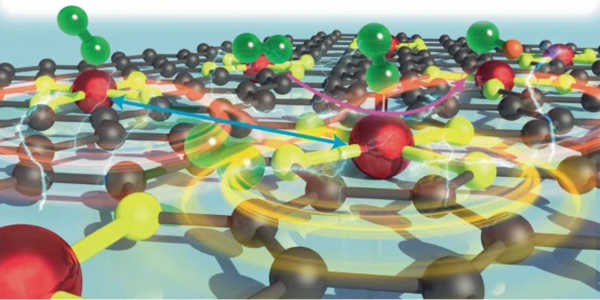
The researchers created a new method to improve the oxygen reduction portion of the chemical reaction in fuel cells, which splits O2 molecules to create water. They did so using a “hydrogel anchoring strategy,” which involves creating densely packed sets of iron atoms that are held in place by a hydrogel polymer. Finding the right formula for spacing these atoms resulted in interactions that allowed them to transform into oxygen reduction catalysts.
Understanding the density and locational dynamics of these iron atoms reveals a previously unknown level of efficiency in this reaction. These findings were recently published in Nature Catalysis in a new paper by the researchers.
The oxygen reduction reaction is possibly the most significant impediment to large-scale fuel cell deployment. The promise of fuel cells is that their potential applications are nearly limitless. They can power systems as large as a utility power station and as small as a laptop computer by using a diverse range of fuels and feedstocks.
Academic researchers from all over the world are working to improve fuel cell capabilities. Other UT Austin engineers are using a variety of approaches to solving key problems in fuel cell development.
However, fuel cells are hampered by slow kinetics in a critical part of the chemical reaction, which limits efficiency. However, the University of Texas at Austin researchers have discovered new dynamics that could supercharge this reaction using iron-based single-atom catalysts.
“It is critical to replace fossil fuels with clean and renewable energy sources in order to address major problems plaguing our society, such as climate change and atmospheric pollution,” said Guihua Yu, an associate professor of materials science in the Cockrell School’s Walker Department of Mechanical Engineering. “Fuel cells are thought to be a highly efficient and sustainable technology for converting chemical to electrical energy; however, they are limited by the sluggish kinetics of the cathodic oxygen reduction reaction. The distance between catalyst atoms was discovered to be the most important factor in maximizing their efficiency for next-generation fuel cells.”
These findings can be applied to a variety of situations, including electrocatalytic reactions. Other types of renewable fuels are included, as well as common chemical products such as alcohols, oxygenates, syngas, and olefin.
The versatility of hydrogen fuel opens up numerous opportunities to replace fossil fuels in various sectors of our economy. It has the potential to provide long-term energy storage for the electric power sector, fuel for heavy-duty transportation, and heat for industrial processes that require high temperatures, such as steel or concrete production. Today, hydrogen is primarily used in the petrochemical, food processing, and fertilizer industries, as well as in automobiles equipped with hydrogen fuel cells.