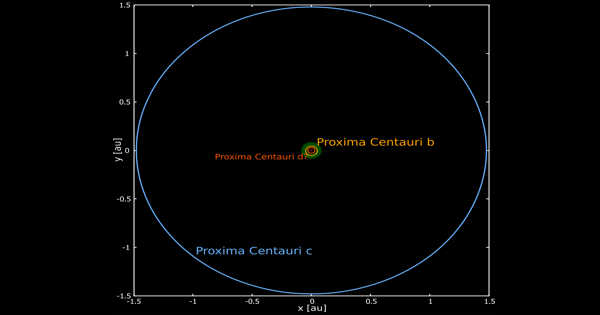
Salicylic acid is an organic compound with the formula HOC6H4CO2H that occurs naturally in plants as a beta hydroxy acid. It’s a crucial organic synthetic raw material that’s used in a variety of applications including medicine, pesticides, dyes, rubber, food, and perfumes. It’s a colorless, bitter-tasting solid that serves as a precursor and metabolite for aspirin (acetylsalicylic acid). Because of its ability to promote exfoliation, this acid has direct anti-inflammatory properties and acts as a topical antibacterial agent.
Its salts, the salicylates, are employed as analgesics and have bacteriostatic, fungicidal, and keratolytic properties. Salicylic acid is used in the pharmaceutical industry to make sodium salicylate, wintergreen oil (methyl salicylate), aspirin (acetylsalicylic acid), salicylic acid amine, and phenyl salicylate. Salicylic acid reduces the production of pro-inflammatory prostaglandins via modulating COX1 enzymatic activity. Salicylate may act as a competitive inhibitor of prostaglandin synthesis. The antirheumatic (nonsteroidal anti-inflammatory) properties of salicylate are due to its analgesic and anti-inflammatory properties.
Salicylic acid is a colorless to light brown substance with no odor. Sinks and eventually dissolves in water. It has sterilizing properties, and a 2.5 percent alcohol solution (called spiritus) is used as a topical medication for tinea manus and tinea pedis. It may also be turned into an ointment. It is a monohydroxybenzoic acid, which is benzoic acid with an ortho hydroxy group. The white willow bark and wintergreen leaves are used to make it.
Salicylic acid works by encouraging epidermal cells to shed more easily, preventing pores from clogging and enabling room for new cell development. It functions as an antiinfective, antifungal, keratolytic, EC 1.11.1.11 (L-ascorbate peroxidase) inhibitor, plant metabolite, algal metabolite, and plant hormone, among other things. It is a salicylate conjugate acid. Salicylic acid contains antipyretic and analgesic properties, as well as the ability to treat rheumatism. Esters can be used as drugs and spices in any combination.
Salicylic acid inhibits the oxidation of uridine-5-diphosphoglucose (UDPG) both competitively and noncompetitively with nicotinamide adenosine dinucleotide. It also prevents the glucuronyl group of uridine-5-phosphoglucuronic acid from being transferred to the phenolic acceptor. Salo is the common name for phenyl salicylate, which is a kind of efficient enteral preservative that is digested into phenol and salicylic acid in the gut. Para aminosalicylic acid is another salicylic acid-related medication.
Salicylates’ wound-healing inhibitory activity is most likely owing to their inhibitory influence on mucopolysaccharide production. Salicylic acid has higher acidity than benzoic acid, and it darkens in color in the light. In the case of iron ions, the purple chelate is formed. When heated to 200 °C, it combines with ferric chloride to create purple, is heat unstable, and is easily decarboxylated to form phenol. Salicylic acid is usually found in the form of methyl in nature, and it may be found in birch bark oil at concentrations of up to 96 percent.
It generates bitter smoke and unpleasant odors when burned to decompose. It can produce the equivalent carboxylic acid ester when it interacts with alcohol or phenol. This substance is poisonous and irritating to the skin, mucous membranes, and bodily tissue protein, making it corrosive. On the other hand, it may be sterilized. High amounts of salicylic acid can enter the bloodstream if high concentrations of salicylic ointment are applied to a substantial percentage of the body surface, necessitating hemodialysis to avoid further problems.
Salicylic acid cures acne by encouraging skin cells to shed more easily, which prevents pores from becoming clogged. Salicylic acid is an active component in numerous shampoos that cure dandruff because of its action on skin cells. The melting point of C6H4 (OH) (COOH) is 157-159 degrees Celsius. It progressively discolored in the light. 1.44 is the relative density. The boiling point is around 211°C/2.67kPa, with sublimation occurring at 76°C. It quickly thermally decomposed to phenol and carbon dioxide at standard pressure.
The amino acid phenylalanine is used to make salicylic acid. It inhibits COX-1 and COX-2 in an irreversible manner, reducing arachidonic acid conversion to prostaglandin and thromboxane precursors. Insoluble in water and soluble in ethanol, ethyl ether, chloroform, benzene, acetone, and turpentine oil. 1 gram salicylic acid was dissolved in 460 milliliters of water, 15 milliliters of boiling water, 2.7 milliliters of ethanol, 3 milliliters of acetone, 3 milliliters of ether, 42 milliliters of chloroform, 135 milliliters of benzene, 52 milliliters of glycerol turpentine, about 60 milliliters of glycerol, and 80 milliliters.
Salicylic acid is a popular medicine for removing the top layer of the skin. Warts, psoriasis, acne vulgaris, ringworm, dandruff, and ichthyosis are all treated with it. It is also utilized as an active component in verrucas removal gels (plantar warts). It’s mostly utilized as a raw material for aspirin feedstock and pesticide aqueous amine and phosphorus products, but it’s also employed in the dye business, refining, and chemical reagents, among other things.
Because salicylic acid is so irritating to the GI mucosa and other tissues, it is rarely utilized systemically; thus, better-tolerated chemical derivatives have been developed for systemic usage. The esterification of the phenolic hydroxyl group of salicylic acid with the acetyl group from acetic anhydride or acetyl chloride produces aspirin (acetylsalicylic acid or ASA). Medicine, spices, colors, rubber auxiliaries, and other fine chemicals all use salicylic acid as a raw ingredient.
In the vacuum ultraviolet spectral region, sodium salicylate is a good phosphor, with approximately flat quantum efficiency for wavelengths between 10 and 100 nm. Antiscorching agents, UV absorbent, foaming agents, and water are all used in the rubber business. Salicylic acid is also utilized as a phenolic resin curing agent, textile printing and dyeing pulp preservatives, synthetic fiber dyeing expansion agent (agent), and other applications. It’s simple to make on a clean surface by spraying a saturated salt solution in methanol and letting it evaporate.
Information Sources: