Selenous acid (or selenious acid) appears to be solid, clear, colorless crystals that are colorless. It’s a chemical compound with the H2SeO3 formula. It decomposes on the basis of heating, water output, and poisonous selenium oxide fumes. Structurally, (HO)2SeO explains it more accurately. It is selenium’s primary oxoacid; selenic acid is the other. It is incompatible with strong reducing agents, organic materials, and finely ground metals and reacts with toxic gaseous hydrogen selenide producing acids upon contact. A non-combustible chemical is selenium acid. It is used as an alkaloid reagent and as an oxidizing agent, and isotopes are used to label radiopharmaceuticals.
Selenium acid decomposes into poisonous and volatile selenium dioxide when heated. Acting as an agent of oxidation. It interacts exothermically with several reducing agents, like hydroiodic acid, sulfuric acid, hyposulfite sodium, hydroxylamine salts, hydrazine salts, hypophosphoric acid, phosphoric acid, but is more readily isolated. By adding selenium dioxide to water, selenous acid is readily formed. The compound can be seen as pyramidal molecules that are intertwined with hydrogen bonds as a crystalline solid. The diprotic acid in the solution is:
H2SeO3 ⇌ H++ HSeO−3 (pKa = 2.62)
HSeO−3 ⇌ H++ SeO2−3 (pKa = 8.32)
In nature, selenous acid is moderately oxidizing but kinetically sluggish. Sodium selenite, a type of selenium, is the acid form. The essential trace element and antioxidant is selenium. It is the regulation of a metabolic enzyme cofactor. It also plays a significant role and has antioxidant properties in preserving the general health of tissue and muscle. Selenium is a part of the enzyme glutathione peroxidase that protects the components of cells from oxidative damage caused by the peroxides created by cell metabolism.
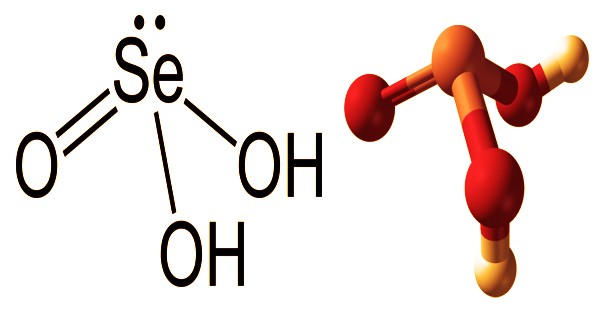
Structural formula, and Ball-and-stick model of Selenous acid
Selenous acid and its salts can penetrate the skin and are capable of causing acute poisoning. It causes skin irritation and burns. Extremely orally poisonous, it is. Dermatitis might be caused by inorganic selenium compounds. Selenium acid, when heated for decomposition, releases poisonous selenium fumes. Protecting and changing the color of steel, especially steel parts on weapons, is the main use. Selenous acid, copper(II) nitrate, and nitric acid are used to change the color of the steel from silver-grey to blue-grey or black in the so-called cold-bluing process. Instead, alternative methods use copper sulfate and phosphoric acid. This method deposits a copper selenide coating and is radically different from other black iron oxide-generating bluing methods. Some of the older razor blades were also made of stainless steel.
Selenious acid is also used as an alkaloid reagent and as an oxidizing agent. In marking radiopharmaceuticals, isotopes are used. It is a trace element that is suggested as a source of selenium in individuals. To sustain the circulatory system, selenium is required. It also protects the muscle and skin tissue of the heart-healthy. Due to its stimulation of antioxidant activity and defense of cell membranes, it may also aid in cancer prevention. Selenium acid is extremely toxic in excessive amounts, like many selenium compounds, and the ingestion of any substantial amount of selenium acid is generally lethal, although it is a permitted dietary source in sufficient quantities.
Information Sources: