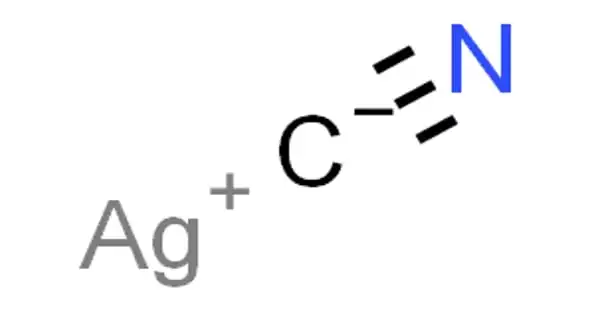Silver cyanide is a chemical compound with the formula AgCN. It is a poisonous compound that forms complex cyanides in silver plating and is obtained as a white curdy precipitate when soluble cyanide is added to the aqueous solutions of a silver salt. It is a white solid that forms when Ag+ solutions are treated with cyanide, which is used in some schemes to recover silver from the solution. Silver cyanide is used in silver plating.
Properties
Silver cyanide appears as a white to gray powder with no odor or taste that darkens when exposed to light. It is water-soluble. It is toxic through skin absorption, ingestion, and inhalation of dust.
- Molecular Weight: 133.89 g/mol
- Appearance: White to Off-White Powder
- Melting Point: 320 °C
- Boiling Point: N/A
- Density: 3.95 g/cm3
- Solubility in H2O: N/A
- Exact Mass: 132.908171
- Monoisotopic Mass: 132.908171
Structure
Silver cyanide has a structure consisting of -[Ag-CN]- chains in which the linear two-coordinate Ag+ ions are bridged by the cyanide ions, which is typical of silver(I) and other d10 ions. This is the same binding mode as the more well-known case of Prussian blue. These chains then pack hexagonally, with adjacent chains offset by +/- one-third of the c lattice parameter. This is the same structure that the high-temperature polymorph of copper(I) cyanide has. In AgCN, the silver-to-carbon and silver-to-nitrogen bond lengths are both 2.09, and the cyanide groups exhibit head-to-tail disorder.

Reactions
When sodium cyanide is added to an Ag+ solution, AgCN precipitates. With the addition of more cyanide, the precipitate dissolves, forming linear [Ag(CN)2](aq) and [Ag(CN)3]2(aq). Silver cyanide dissolves in solutions containing other ligands, such as ammonia or tertiary phosphines. When silver cyanides react with other anions, they form structurally complex materials. Some silver cyanides emit light.
Silver cyanide is quickly decomposed by acids, releasing hydrogen cyanide, a flammable poison gas. Aqueous ammonia, dilute boiling nitric acid, aqueous potassium cyanide solution, and aqueous sodium thiosulfate solutions are all soluble. Under certain conditions, it is prone to explosive instability or violent oxidation. A violent explosion can result from fusion with metal chlorates, perchlorates, nitrates, or nitrites. At normal temperatures, fluorine and silver cyanide react violently.
Uses
Both AgCN and KAg(CN)2 have been used in silver-plating solutions since at least 1840 when the Elkington brothers patented their recipe for a silver-plating solution. A typical, traditional silver-plating solution would contain 15-40 g·L-1 KAg(CN)2, 12-120 g·L-1 KCN, and 15 g·L-1 K2CO3.
Health Hazards
If inhaled, swallowed, or absorbed through the skin, this highly toxic substance can be fatal. Any skin contact should be avoided. Contact or inhalation effects may be delayed. Fire can emit irritant, corrosive, and/or toxic gases. Firefighting or dilution water runoff can be corrosive and/or toxic, resulting in pollution.