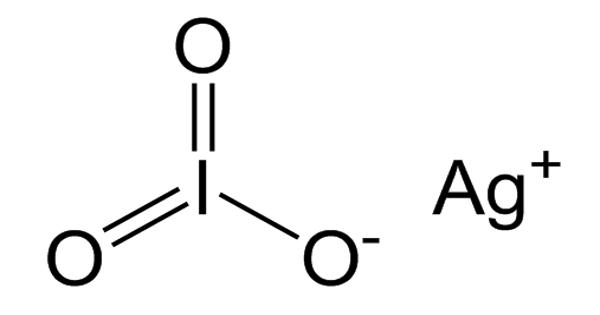
Silver iodate (AgIO3) is a light-sensitive, white crystal composed of silver, iodine, and oxygen. It is an odorless white solid. Unlike most metal iodates, it is practically insoluble in water. It is an inorganic compound that is highly photosensitive (reacts when exposed to light). It is a compound that darkens on exposure to light and is used in photography, rainmaking, and medicine.
Properties
The compound is a bright yellow solid, but samples almost always contain impurities of metallic silver that give a gray coloration. The silver contamination arises because AgI is highly photosensitive. It generally exists with small amounts of silver metal as impurity, so is found as a greyish yellow solid.
- Molecular Weight: 282.7709
- Appearance: solid
- Melting Point: N/A
- Boiling Point: N/A
- Density: 5.525 g/cm3
- Solubility in H2O: N/A
- Exact Mass: 281.794305
AgI is insoluble in water and organic solvents, and dissolves in salt or basic solutions such as sodium chloride and ammonium hydroxide. AgI is highly photosensitive and darkens when exposed to light due to the formation of metallic silver.

Production
Silver iodate can be obtained by reacting silver nitrate (AgNO3) with sodium iodate or potassium iodate. The by-product of the reaction is sodium nitrate.
It is prepared by the reaction of potassium iodide with an aqueous solution of silver nitrate, and the insoluble silver iodide precipitates out of solution. However, it is obtained as a mixture of the alpha and beta phases, which can be subsequently separated.
AgNO3 + KI → AgI + KNO3
When AgI is dissolved in a concentrated aqueous solution of silver nitrate (AgNO3) and then diluted with water, the alpha-AgI is exclusively formed. When AgI is dissolved in hydroiodic acid (HI), and then diluted with water, the beta-AgI
Alternatively, it can be created by the action of iodine in a solution of silver oxide.
Uses
Silver iodate is used to detect traces of chlorides in blood. It is a crystalline material often used for weather modification applications such as cloud seeding or anti-hail systems. Other uses serving as an antiseptic material and a photosensitive material in photography.
Health Hazard
Contact with eyes causes irritation. Exposure to high levels of silver over a long period of time can result a gray or blue-gray discoloration of the skin and other tissues known as argyria. If continued for a long period, ingestion or inhalation of silver compounds can cause permanent discoloration of the skin. While this condition is permanent, it is thought to only be a cosmetic problem that is not otherwise harmful to health.