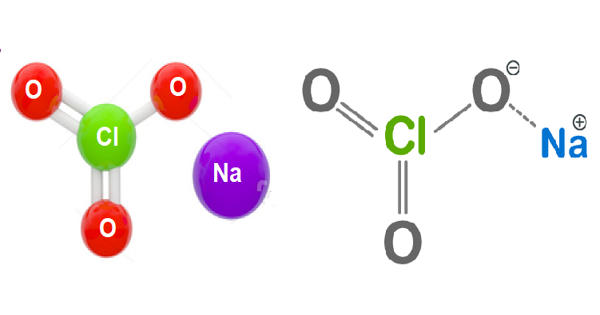
Sodium chlorate is a white crystalline inorganic compound produced from salt (sodium chloride) and water in a reaction with electricity. It is an inorganic compound with the chemical formula NaClO3. It is a white crystalline powder that is readily soluble in water. It is an inorganic sodium salt that has chlorate as the counter-ion. It is hygroscopic. It decomposes above 300 °C to release oxygen and leaves sodium chloride.
Several hundred million tons are produced annually, mainly for applications in bleaching pulp to produce high brightness paper. It is a strong oxidizing agent that is readily soluble in water; it is a versatile product that is used in a variety of applications.
Properties
Sodium chlorate appears as an odorless pale yellow to white crystalline solid. It is appreciably soluble in water and heavier, so may be expected to sink and dissolve at a rapid rate.
- Molecular Weight: 106.44
- Appearance: Colorless to white crystals or powder
- Melting Point: 248-261 °C
- Boiling Point: N/A
- Density: 2.5 g/cm3
- Solubility in H2O: N/A
- Exact Mass: 105.943366

Synthesis
The manufacture of sodium chlorate is a potential source of perchlorate because perchlorate is generated as an unintended by-product in the electrolysis process. Industrially, sodium chlorate is produced by the electrolysis of a hot sodium chloride solution:
NaCl + 3 H2O → NaClO3 + 3 H2
Although it is not itself flammable, the solid product and even 30% solutions in water are powerful oxidizing agents. This reaction progresses in heat (at least 70 °C), and controlled pH. In lower temperature or with high pH another reaction progresses:
2 NaCl + H2O → NaClO + NaCl + H2
Sodium chlorate is used as herbicide, mainly as a defoliant. The sodium chlorate process is not to be confused with the Chloralkali process, which is an industrial process for the electrolytic production of sodium hydroxide and chlorine gas. Crystals of sodium chlorate can be obtained from solution and crystal chirality can be recognized by observation with a microscope under polarized light illumination.
It is noncombustible but it can accelerate the burning of surrounding combustible materials. This can easily occur if the material should dry out. Contact with strong sulfuric acid may cause fires or explosions.
Uses
The main commercial use for sodium chlorate is for making chlorine dioxide (ClO2). The largest use for sodium chlorate is for the generation of chlorine dioxide, which is used for bleaching chemical pulp. The largest application of ClO2, which accounts for about 95% of the use of chlorate, is in bleaching of pulp. All perchlorate compounds are produced industrially by the oxidation of solutions of sodium chlorate by electrolysis. In the mining industry, sodium chlorate is used during the extraction process for a variety of metals. It is also used as an intermediate in the generation of ferric chloride which is used as a flocculant in many industrial water treatment applications. It is also used in the manufacture of dyes, explosives and matches.
Information Source: