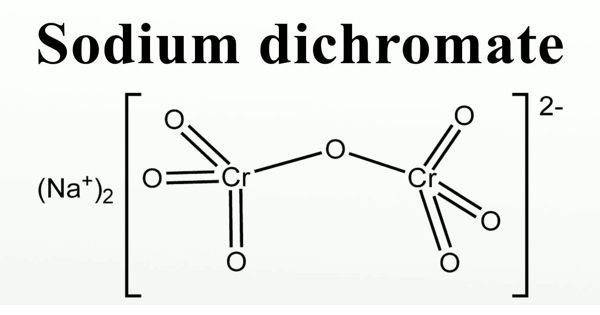
Sodium dichromate is the inorganic compound with the formula Na2Cr2O7. It is also called Bichromate of soda or Disodium dichromate or Sodium dichromate (VI). It is an orange to red-colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. Usually, however, the salt is handled as its dihydrate Na2Cr2O7·2H2O. It is highly corrosive and is a strong oxidizing agent.
Virtually all chromium ore is processed via conversion to sodium dichromate and virtually all compounds and materials based on chromium are prepared from this salt. This substance is mainly used to produce other chromium compounds but is also used in drilling muds, in metal treatments, in wood preservatives, in the production of dyes and organic chemicals, and as a corrosion inhibitor.
In terms of reactivity and appearance, sodium dichromate and potassium dichromate are very similar. These are inorganic sodium salt. The sodium salt is, however, around twenty times more soluble in water than the potassium salt (49 g/L at 0 °C) and its equivalent weight is also lower, which is often desirable. It is odorless and dissolves in water, methanol as well as ethanol. It is widely used as a corrosion inhibitor.

Properties
- Molecular weight: 261.97 g/mol (anhydrous)
- Density: 2.52 g/dm3
- Melting point: 356.7 °C
- Boiling point: 400 °C
Reactions
Sodium dichromate appears as a red or red-orange crystalline solid. Dichromate and chromate salts are oxidizing agents. For the tanning of leather, sodium dichromate is first reduced with sulfur dioxide.
In the area of organic synthesis, this compound oxidizes benzylic and allylic C-H bonds to carbonyl derivatives. For example, 2,4,6-trinitrotoluene is oxidized to the corresponding carboxylic acid.
Secondary alcohols are oxidized to the corresponding ketone, e.g. menthol to menthone; dihydrocholesterol to cholestanone:
3 R2CHOH + Cr2O72- + 2 H+ → 3 R2C=O + Cr2O3 + 4 H2O
Relative to the potassium salt, the main advantage of sodium dichromate is its greater solubility in water and polar solvents like acetic acid.
Safety
Like all hexavalent chromium compounds, sodium dichromate is carcinogenic. Sodium dichromate primarily affects the respiratory system causing ulcerations, shortness of breath, bronchitis, pneumonia, and asthma but can also affect the gastrointestinal tract, liver, kidneys, and immune system. The compound is also corrosive and exposure may produce severe eye damage or blindness. Swallowing causes diarrhea and vomiting. When exposed to eyes and skin it results in local irritation and dermatitis. Human exposure further encompasses impaired fertility, heritable genetic damage, and harm to unborn children.
Uses
- Sodium dichromate is a primary material in the manufacturing of chromium chemicals.
- It is used as textile dyes and leather tanning.
- It is used as an oxidizing agent in the manufacturing of synthetic organic chemicals.
- It is used in the refining of petroleum.
- It is used as an insecticide and fungicide.
Information Source: