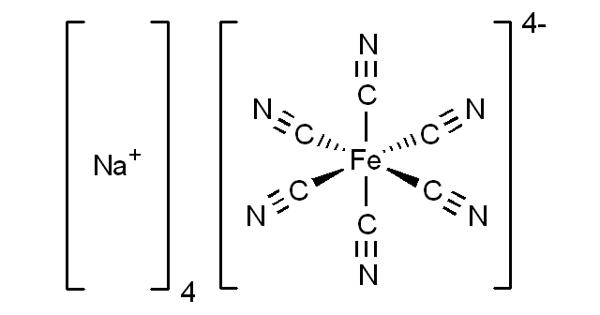
Sodium ferrocyanide is an odorless yellow solid. It is the sodium salt of the coordination compound of the formula [Fe(CN)6]4-. In its hydrous form, Na4Fe(CN)6 • 10H2O (sodium ferrocyanide decahydrate), it is sometimes known as yellow prussiate of soda. Ferrocyanide complexes are stable and practically nontoxic because of the strong bonding between iron and cyanide. It is a sodium-iron compound used as an anti-caking agent, electroplating additive, and petroleum refining reagent.
Properties
It is a yellow crystalline solid that is soluble in water and insoluble in alcohol. It is a yellow crystalline salt similar to potassium ferrocyanide and used in making iron blue pigments, blueprint paper, and dyes. The yellow color is the color of ferrocyanide anion.
- Molecular Weight: 303.91
- Appearance: Yellow crystals
- Melting Point: 435 °C
- Boiling Point: N/A
- Density: 1.485 g/cm3
- Solubility in H2O: 17.6 g/100 mL (20 °C)
- pH: 9.2 – 9.4
- Crystal Phase / Structure: Monoclinic
- Exact Mass: 303.912459

Uses
- When combined with iron, it converts to a deep blue pigment called Prussian blue. It is used as a stabilizer for the coating on welding rods.
- In the petroleum industry, it is used for removal of mercaptans.
- It is used as oxidant, food additive, explosive and chemical reagent. Also used as additive for preventing agglomeration in melt snow in winter.
- In the EU, ferrocyanides (E 535–538) were, as of 2018, solely authorised in two food categories as salt substitutes. Kidneys are the organ for ferrocyanide toxicity.
- It is used in producing iron blue and potassium ferricyanide, surface corrosion protecting for tannage and metal, carburization treating for carbon steel, removing iron in pharmaceutical producing.
Production
Sodium ferrocyanide is produced industrially from hydrogen cyanide, ferrous chloride, and calcium hydroxide, the combination of which affords Ca2[Fe(CN)6] • 11H2O. It is a fine, (light) yellow crystalline. A solution of this salt is then treated with sodium salts to precipitate the mixed calcium-sodium salt CaNa2[Fe(CN)6], which in turn is treated with sodium carbonate to give the tetrasodium salt. The compound has widespread use as an anti caking agent in salt, citric acid production and in pigments.
Toxicity
Despite the presence of the cyanide ligands, sodium ferrocyanide has low toxicity. The ferrocyanides are less toxic than many salts of cyanide, because they tend not to release free cyanide. However, like all ferrocyanide salt solutions, addition of an acid can result in the production of hydrogen cyanide gas, which is toxic.
Information Source: