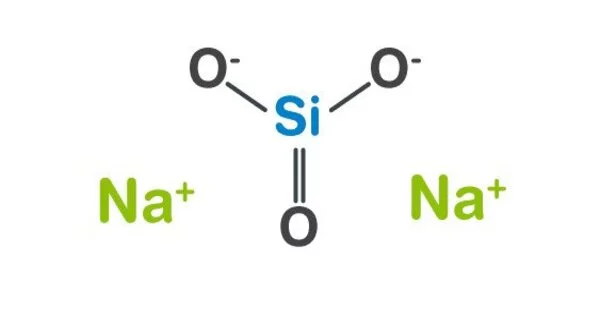
The term sodium silicate refers to chemical compounds with the formula Na2xSiyO2y+x or (Na2O)x•(SiO2)y, such as sodium metasilicate Na2SiO3, sodium orthosilicate Na4SiO4, and sodium pyrosilicate Na6Si2O7. Anions are frequently polymeric. These compounds are generally colorless, transparent solids or white powders that are soluble in varying amounts of water.
Sodium silicate is also the technical and common name for a mixture of such compounds, primarily metasilicate, which is also known as waterglass, water glass, or liquid glass. The product has a wide range of applications, including cement formulation, passive fire protection, textile and lumber processing, refractory ceramics manufacturing, adhesives, and silica gel production. The commercial product, available in water solution or in solid form, is often greenish or blue owing to the presence of iron-containing impurities.
Properties
Sodium silicates are white powders or colorless glassy or crystalline solids. Except for the most silicon-rich ones, they dissolve easily in water and produce alkaline solutions.
In neutral and alkaline solutions, sodium silicates are stable. Silicate ions react with hydrogen ions in acidic solutions to form silicic acids, which decompose into hydrated silicon dioxide gel. When heated to remove the water, the result is a hard translucent substance known as silica gel, which is widely used as a desiccant. It can withstand temperatures of up to 1100 degrees Celsius.
- Solubility: It is highly soluble in water, forming a viscous, alkaline solution.
- Alkalinity: It solutions are highly alkaline due to the presence of sodium hydroxide (NaOH).
- Adhesive and binding properties: It is known for its adhesive and binding properties when it comes into contact with certain materials, such as paper, cardboard, fabrics, and cement.
- Fire resistance: It has fire-resistant properties, making it useful as a flame retardant.
- pH buffering: It solutions have the ability to buffer or stabilize pH levels.
Production
Sodium silicate solution can be prepared in a reactor by treating a mixture of silica, water, and caustic soda with hot steam. The reaction is as follows:
2x NaOH + SiO2 → (Na2O)x·SiO2 + x H2O
Sodium metasilicate can also be produced by dissolving silica SiO2 in molten sodium carbonate (Na2CO3) at a temperature of 851 °C
x Na2CO3 + SiO2 → (Na2O)x·SiO2 + CO2
Application
Sodium silicate is used as an adhesive or binder in a variety of applications such as making cardboard, paperboard, and fiber drums. It can also be used in the production of refractory materials, foundry molds, and as a binder in briquettes for solid fuel.
The alkaline nature of sodium silicate makes it useful in water treatment processes. It is used in the formulation of detergents and cleaning products due to its ability to provide alkalinity and improve cleaning performance.
It can be also used as an additive in cement and concrete formulations. It acts as a water reducer, improving workability and strength development in the final product. It is sometimes used in automotive and metal repair applications.