Solderability refers to how easy a metal is to solder to. It is defined as the ability of a metal to be wetted by molten solder. The solderability of a substrate is a measure of the ease with which a soldered joint can be made to that material. Some metals like copper and tin are easy to solder to. Other metals like brass and steel are difficult to solder to. Good solderability requires wetting (low contact angle) of the substrate by the solder.
Solderability of metals
Solderability varies depending on the type of solder alloy under discussion. The discussion that follows applies only to unspecified electronic solders. Solderability when using lead-free alloys can differ significantly from solderability when using lead-based alloys. The majority of the materials used in micro-welding applications are either plated or coated to improve corrosion resistance, solderability, or for better absorption of the laser energy.
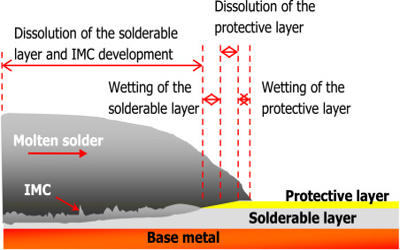
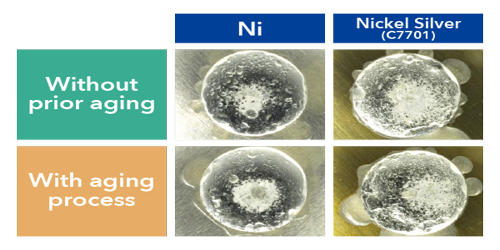
Fig: Common Solderability of Base Metals
Noble metals may be easy to solder but they have brittle joints. A material exhibits good solderability when its surface is able to form a bond with the molten solder. Most pure metals and alloys can readily bond with solder, however, surface oxidation, impurities, and the formation of intermetallic compounds can inhibit this bond. The metals in the good category require a large amount of heat therefore oxidation is an issue. To overcome this a flux is required. For carbon steel, low alloy steel, zinc, and nickel the presence of sulfur creates a brittle joint; lower temperatures are used to minimize this problem. The oxides on the surface of aluminum cause wetting issues and special solders must be used to prevent galvanic corrosion issues. Common methods of alloy fabrication include sol-gel, chemical methods, vapor deposition techniques, melting and casting, severe plastic deformation, and high-energy ball milling (HEBM).
Stainless steel and high alloy steel have a low solderability because the chromium alloying element creates oxides that require aggressive fluxes. The thickness and adherence of the oxide layer are dependent on the metal or alloy. Solder fluxes, containing reducing agents, are employed to break down the oxide layer to allow soldering. When metal oxidizes it becomes harder to solder. Due to oxidation, an easy to solder metal can become hard to solder. The only way that the final category of metals can be soldered is by pre-plating them in a metal that is solderable. Thicker and more stable oxides require more active fluxes to allow solderability. Intermetallics have very stable oxides and therefore require the most active fluxes to obtain good solderability.