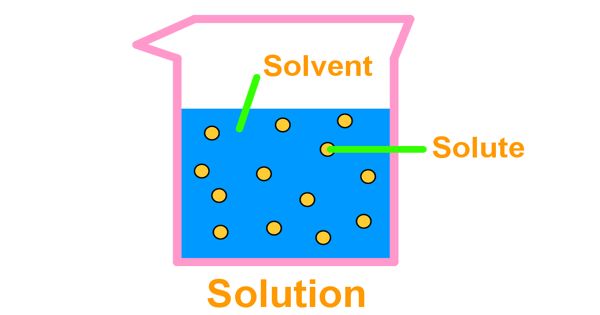
A solution is a homogeneous mixture of two or more substances. In chemistry, a solution is a homogeneous mixture of two or more substances. The substances that are dissolved are called solutes. The substance the solutes are dissolved in is called the solvent. Sugar syrup is a solution where sugar is dissolved in water using heat. Here, water is the solvent and sugar is the solute.
A solution is a homogeneous mixture of two or more components in which the particle size is smaller than 1 nm. Common examples of solutions are sugar in water and salt in water solutions, soda water, etc.
In chemistry, solution means a homogenous mixture of two or more substances in relative amounts that can be varied continuously up to what is called the limit of solubility. An example from everyday experience is a solid like salt or sugar (which are crystalline solids), dissolved in a liquid (like water). Gases can dissolve in liquids. An example is carbon dioxide or oxygen in the water. Liquids may dissolve in other liquids and gases in other gases. Air, for example, is a solution consisting chiefly of oxygen and nitrogen with trace amounts of several other gases, and brass is a solution composed of copper and zinc.
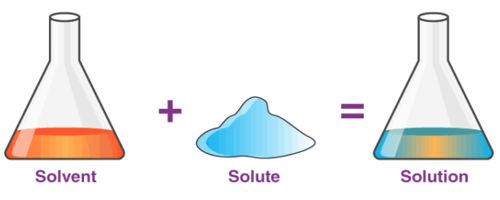
The amount of solute added to the solvent determines the concentration of the solution. The amount of solute that can be dissolved in solvent is called its solubility. For example, in a saline solution, salt is the solute dissolved in water as the solvent. The solution with the large amount of solute is called a concentrated solution; the solution with less solute is called a dilute solution.
Characteristics of a Solution –
- A solution consists of a homogeneous mixture.
- A solution is composed of one phase (e.g., solid, liquid, gas).
- Particles in a solution are not visible to the naked eye.
- A solution does not scatter a light beam.
- Components of a solution cannot be separated using simple mechanical filtration.
Properties of Solution
- It is a homogeneous mixture.
- Its particles are too tiny and have a diameter less than 1 nm.
- The particles are not visible to naked eyes.
- Particles don’t scatter a beam of light passing through it and hence the path of the light is not visible.
- Solutes are inseparable from the mixture and do not sediment. A solution is stable.
- The components of a mixture cannot be separated using filtration.
For solutions with components in the same phase, the substances present in lower concentration are solutes, while the substance present in highest abundance is the solvent. Examples of solid solutions are alloys and some minerals. For example, brass is an alloy of copper and zinc.
Information Source: