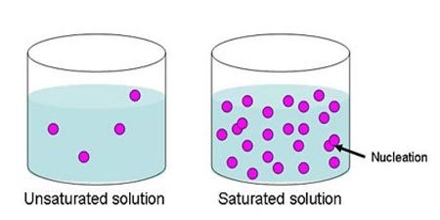
Prime purpose of this lecture is to present on Solutions and Solubilities. Solution is a homogeneous mixture containing particles the size of a typical ion or covalent molecule. (0.1–2.0 nm in diameter). Solubilities means Colloid, Solute, Solvent etc. Colloidis a homogeneous mixture containing particles with diameters in the range 2–500 nm. Solute is the dissolved substance in a solution and Solvent is the major component in a solution.
Solutions and Solubilities