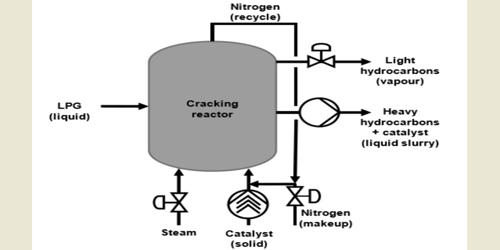
Steam Cracking is the cracking of petroleum hydrocarbons, at high temperatures in the presence of steam, in order to produce ethylene, propylene, and other light alkenes. It is a petrochemical process in which saturated hydrocarbons are broken down into smaller, often unsaturated, hydrocarbons. It is the principal industrial method for producing the lighter alkenes, including ethane (or ethylene) and propene (or propylene). We depend largely on crude, the gases associated with it, and natural gas (main methane) as the source of liquid fuels (petrol, diesel) and the feedstock for the chemical industry. Petrol and other fuels are produced from it using fractional distillation. Cracking is used to convert long alkanes into shorter, more useful hydrocarbons.
Steam Cracking is a high-temperature cracking of petroleum hydrocarbons in the presence of steam. Steam cracker units are facilities in which a feedstock such as naphtha, liquefied petroleum gas (LPG), ethane, propane, or butane is thermally cracked through the use of steam in steam cracking furnaces to produce lighter hydrocarbons. The most valuable fractions for the chemical industry, and for producing petrol, are liquefied petroleum gas (LPG), naphtha, kerosene, and gas oil. These are treated in several ways including cracking, isomerization and reforming. The propane dehydrogenation process may be accomplished through different commercial technologies. The main differences between each of them concerns the catalyst employed, design of the reactor and strategies to achieve higher conversion rates.
Steam cracking is the main method of breaking down large molecules of hydrocarbons, in which a gaseous or liquid hydrocarbon is diluted with steam and then heated. Cracking is a reaction in which larger saturated hydrocarbon molecules are broken down into smaller, more useful hydrocarbon molecules, some of which are unsaturated:
- the original starting hydrocarbons are alkanes
- the products of cracking include alkanes and alkenes, members of a different homologous series
Olefins are useful precursors to myriad products. Steam cracking is the core technology that supports the largest-scale chemical processes, i.e. ethylene and propylene.
Cracking is important for two main reasons:
- It helps to match the supply of fractions with the demand for them.
- It produces alkenes, which are useful as feedstock for the petrochemical industry.