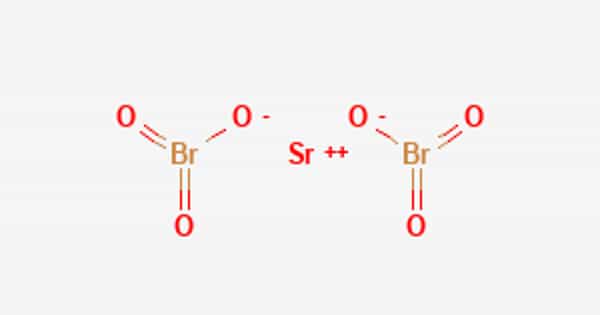
Strontium bromate is a chemical that is rarely used in laboratories or industries. This salt is a moderately strong oxidizing agent and is soluble in water. Strontium bromate is a moderately strong oxidizing agent and is soluble in water (30.9 g/100 ml at 20°C). It is also toxic when ingested and irritates the skin and respiratory tract when in contact with or inhaled. In the laboratory or in industry, strontium bromate is rarely used. It is commercially available but in limited quantities.
Properties
It is colorless or yellowish crystals, lustrous powder, hygroscopic. It is soluble in water. Loses water at 120C, decomposes at 240C. Chemically this salt is soluble in water and is a moderately strong oxidizing agent.
- Molecular Weight: 343.4244
- Appearance: Crystalline
- Melting Point: 240 °C
- Boiling Point: N/A
- Density: N/A
- Solubility in H2O: N/A
- Exact Mass: 343.709731
Preparation
Strontium Bromate can be prepared by reacting strontium hydroxide with sodium bromate or by reacting strontium sulfate with barium bromate:
Sr(OH)2 + 2NaBrO3 →Sr(BrO3)2 + 2NaOH
SrSO4 (aq)+ Ba(BrO3)2 (solid)→Sr(BrO3)2 (aq)+ BaSO4 (solid)
Of the two methods, the latter seems to be more stable since the temperature can be as high as 80°C in solution.
The usual product is a monohydrate, Sr(BrO3)2·H2O. Sr(BrO3)·H2O occurs as small, colorless but lustrous, monoclinic crystals which are prismatic in nature. It is somewhat soluble in water and loses its water of hydration if heated to about 110°C. It decomposes at 240°C forming strontium bromide and oxygen.
Strontium bromate is approximately 40 times more water-soluble than barium bromate. The increased solubility and stability of strontium MEDTA chelate tend to keep strontium in solution. The main advantage of this method over the precipitation of barium chromate to separate Sr from Ba is in the preparation of barium compounds. Before proceeding, barium is usually separated from the chromate.
Hazard
Strontium bromate is toxic if ingested and irritates the skin and respiratory tract if come into contact with or inhaled, respectively. Its chemical formula is Sr(BrO3)2.