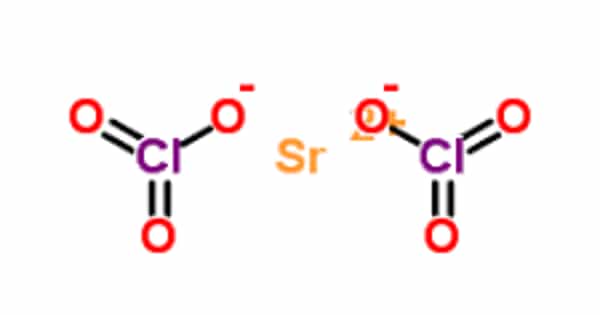
Strontium chlorate is a chemical compound with the formula Sr(ClO3)2. It has high oxidizing power. It appears as a moist solid or semi-solid slurry of white crystals. It creates a flammable mixture with combustible materials (can be ignited by friction). Such mixtures may be explosive.
Contact with concentrated sulfuric acid can result in fires or explosions. When mixed with ammonium salts, spontaneous decomposition and ignition may occur. In the presence of strong acid, this compound emits explosive chlorine dioxide gas; heating with a dibasic organic acid in the presence of moisture emits chlorine dioxide and carbon dioxide. Mixtures with ammonium salts, powdered metals, silicon, sulfur, or sulfides are readily ignited and potentially explosive.
Properties
Strontium chlorate has a molecular weight of 254.52 g/mol. It is a white solid with a melting point of 120°C and a density of 3.152 g/cm3. It is soluble in water (174 g/100 ml at 20°C). These white, water-soluble crystals decompose at 120°C. It is used in pyrotechnics and tracer bullets.
- Formula Weight: 254.52
- Melting point: 120° with decomposition and evolution of O2
- Density: 3.15

Preparation
Strontium chlorate is made by heating a strontium hydroxide solution and adding chlorine to it, followed by crystallization. Chlorine has no effect on dry Sr(OH)2, but it does convert the hydrate [Sr(OH)2•8H2O] into chloride and chlorate, as well as a small amount of strontium hypochlorite.
Strontium chlorate can be produced by the Liebig method which consists of passing chlorine gas through a solid such as Sr(OH)2:
6Sr(OH)2+ 6Cl2 → 5SrCl2 + Sr(CIO3)2 + H2O.
However, separating the two salts remains problematic since both are soluble in water. Strontium chloride solubility is 42.9 g/100 ml of water at 20°C while that of strontium chlorate is 174.9 g/100 ml of water at 20°C.
Reactivity Profile
Strontium chlorate is a powerful oxidizer. combines with combustible materials to form a flammable mixture (can be ignited by friction). Such mixtures have the potential to be explosive. Sulfuric acid can cause fires or explosions if it comes into contact with it. When combined with ammonium salts, it is possible that spontaneous decomposition and ignition will occur. In the presence of strong acid, it produces explosive chlorine dioxide gas; in the presence of moisture, heating with a dibasic organic acid produces chlorine dioxide and carbon dioxide. Ammonium salts, powdered metals, silicon, sulfur, or sulfides are easily ignited and potentially explosive in mixtures.
Hazard – When in contact with organic materials, there is a risk of an explosion; it is extremely sensitive to shock, heat, and friction; and it is a strong oxidizing agent.
It is toxic; inhaling, ingesting, or coming into contact (skin, eyes) with the vapors, dust, or substance can result in severe injury, burns, or death. Fire can emit irritants and/or toxic gases. Toxic fumes or dust can build up in confined spaces (basements, tanks, hopper/tank cars, and so on). Pollution may occur as a result of runoff from firefighting or dilution water.