Sulfurous acid (H2SO3) is a sulfur oxoacid. It is the conjugate acid of a hydrogensulfite. It is a tautomer of a sulfonic acid. It is also called Sulfur dioxide solution or dihydrogen trioxosulfate or trioxosulfuric acid. It is an intermediate species to produce acid rain from sulfur dioxide (SO2).
There is no evidence that sulfurous acid exists in solution, but the molecule has been detected in the gas phase. The conjugate bases of this elusive acid are, however, common anions, bisulfite (or hydrogen sulfite), and sulfite. Sulfurous acid is an intermediate species in the formation of acid rain from sulfur dioxide.
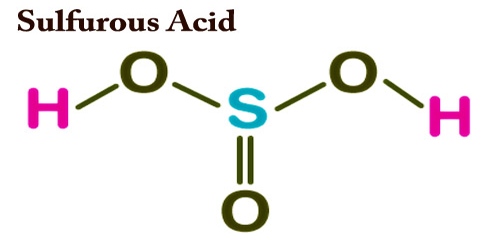
The chemical formula of sulfurous acid is H2SO3 and its molar mass is 82.07 g/mol. Its chemical structure is shown below. It consists of a sulfur atom having two single bonds with hydroxyl groups and one double bond with oxygen.
According to Raman spectra of SO2 solutions shows that the intensities of the signals are consistent with the equilibrium as follows:
SO2 + H2O ⇌ HSO−3 + H+
Ka = 1.54×10−2; pKa = 1.81.
Sulfurous acid appears as a colorless liquid with a pungent burning sulfur odor. Corrosive to metals and tissue. It is found in nature as an intermediate in the formation of acid rain, by the reaction of sulfur dioxide with atmospheric moisture. Sulfurous acid is not usually available in its acid form, but more commonly prepared as its sodium or potassium salts. In the below reaction, sodium sulfate is added to a solution of sulfur dioxide in water, to give the stable sodium bisulfite salt as a product.
Na2SO3 + H2O + SO2 → 2NaHSO3
Sulfurous acid is a toxic, corrosive, and non-combustible compound. Inhaling, ingesting or skin contact with Sulfur dioxide solution causes severe injury which leads to death. In its molten form, it can cause severe burns to eyes and skin. Therefore avoid skin contact with this compound. On this compound, it liberates corrosive, toxic, and irritating gases.
Aqueous solutions of sulfur dioxide, which sometimes are referred to as sulfurous acid, are used as reducing agents and as disinfectants, as are solutions of bisulfite and sulfite salts. They are oxidized to sulfuric acid or sulfate by accepting another oxygen atom. Sulfurous acid and its salts are used as powerful reducing agents and disinfecting agents. It is also used as a mild bleaching agent for applications having chlorine sensitive materials.
Sulfurous acid can affect us by breathing in and moving through our skin. Sulfurous acid is a corrosive substance, so contact with potential eye damage can seriously irritate so burn the skin and eyes. Breathing Sulfurous Acid can irritate the neck and throat. Sulfurous acid itself is not commercially available as a free agent, and thus its health hazards are not severe on its own.
However, it readily decomposes to release sulfur dioxide gas, which is toxic. Moreover, it forms sulfuric acid when exposed to air, which is toxic, strong, and corrosive acid.
Information Sources: