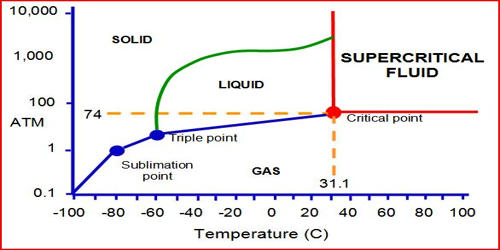
Supercritical carbon dioxide (sCO2) is a supercritical fluid is a substance at a temperature and pressure above its critical temperature and pressure. It is a fluid state of carbon dioxide where it is held at or above its critical temperature and critical pressure. The critical point represents the highest temperature and pressure at which the substance can exist as a vapor and liquid in equilibrium. It is an established precision cleaning technique with applications in many different industries. The gas-like viscosity and the liquid-like density of CO2 are the key characteristics that allow the process to be tuned to the application. It has the unique ability to diffuse through solids like a gas and dissolve materials like a liquid.
Carbon dioxide usually behaves as a gas in air at standard temperature and pressure (STP), or as a solid called dry ice when frozen. If the temperature and pressure are both increased from STP to be at or above the critical point for carbon dioxide, it can adopt properties midway between a gas and a liquid. Carbon dioxide usually behaves as a gas in air or as a solid called dry ice when frozen. More specifically, it behaves as a supercritical fluid above its critical temperature (304.13 K, 31.0°C, 87.8°F) and critical pressure, expanding to fill its container like a gas but with a density like that of a liquid. Above its critical temperature and pressure, it behaves like a supercritical fluid and can adopt properties midway between a gas and a liquid. Supercritical carbon dioxide could be an alternative solvent for catalytic liquid-phase hydrogenation.
Supercritical CO2 is becoming an important commercial and industrial solvent due to its role in chemical extraction in addition to its low toxicity and environmental impact. While conventional power plant cycles produce power from turbines using water or steam as the working fluid, supercritical carbon dioxide (sCO2) cycles use CO2 that is in a supercritical state—at a temperature and pressure above its critical point where liquid and gas phases are not distinguishable. The relatively low temperature of the process and the stability of CO2 also allows most compounds to be extracted with little damage or denaturing. In addition, the solubility of many extracted compounds in CO2 varies with pressure, permitting selective extractions.
Supercritical carbon dioxide is utilized as a coloring medium to color manufactured filaments and textures. Chemical processing of textile materials in supercritical carbon dioxide has been identified as one of the alternatives to water-based conventional processes. Supercritical carbon dioxide can also be used as a more environmentally friendly solvent for dry cleaning compared to more traditional solvents such as hydrocarbons and perchloroethylene. It is widely used in many industrial applications as a process or heat transfer fluid.