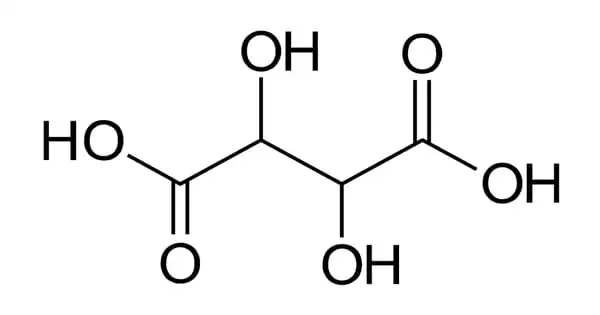
Tartaric acid is an organic acid found in a variety of vegetables and fruits, including bananas and grapes, as well as bananas, citrus, and tamarinds. It is a white, crystalline organic acid found in a variety of fruits, most notably grapes, but also bananas, tamarinds, and citrus. Its salt, potassium bitartrate, also known as “cream of tartar,” forms naturally during the fermentation process. It is also known as Racemic acid or 2,3-dihydroxysuccinic acid. Its purpose is to produce carbon dioxide.
It is frequently combined with sodium bicarbonate and sold as baking powder, which is used as a leavening agent in food preparation. The acid is added to foods as an antioxidant E334 and imparts their distinct sour flavor. It is a crystalline white diprotic aldaric acid.
Tartaric acid is a naturally occurring raw material that can be used in organic chemical synthesis. Tartaric acid is a dihydroxyl derivative of succinic acid and is an alpha-hydroxy-carboxylic acid with diprotic and aldaric acid properties.
Properties
Tartaric acid is the acid most prescribed for modifications to the must. It is a stronger acid than malic and citric acid, and less susceptible to microorganism breakdown during alcoholic and malolactic fermentations. Tartaric acid is a potassium salt that tends to precipitate.
- Molecular Weight/ Molar Mass: 150.087 g/mol
- Density: 1.79 g/mL
- Boiling Point: 275 °C
- Melting Point: 171 to 174 °C
- Form: Cristal
- Color: White

History
Winemakers have known about tartaric acid for centuries. The chemical extraction process, on the other hand, was developed in 1769 by the Swedish chemist Carl Wilhelm Scheele.
Tartaric acid was crucial in the discovery of chemical chirality. Jean Baptiste Biot discovered this property of tartaric acid in 1832 when he noticed its ability to rotate polarized light. In 1847, Louis Pasteur expanded on this work by studying the shapes of sodium ammonium tartrate crystals, which he discovered to be chiral. Pasteur was the first to produce a pure sample of levotartaric acid by manually sorting the differently shaped crystals.
Applications
Tartaric acid and its derivatives have a wide range of applications in the pharmaceutical industry. It has been used, for example, in the production of effervescent salts in conjunction with citric acid to improve the taste of oral medications.
Tartaric acid has a number of industrial applications. Metal ions such as calcium and magnesium have been observed to chelate in the acid. As a result, the acid has been used as a chelating agent in the farming and metal industries, respectively, for complexing micronutrients in soil fertilizer and cleaning metal surfaces made of aluminum, copper, iron, and alloys of these metals.
- It is used to improve the taste of oral medications,
- It is used in recipes as a leavening agent along with baking soda,
- It is used as an antioxidant,
- It is used in foods to give a sour taste,
- It is used to make silver mirrors,
- It is used in the tanning of leather.