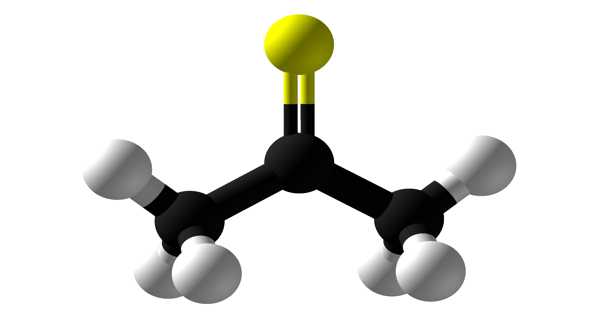
Thioacetone has the chemical formula (CH3)2CS and is an organosulfur compound. It is a compound that has received little attention. It is a volatile orange or brown substance that can only be isolated at low temperatures. At low temperatures, the compound has been isolated as an orange or brown liquid. Thioacetone readily converts to a polymer and a trimer, trithioacetone, above 20°C(4°F). Thioacetone has a very strong, unpleasant odor, so it is known as the worst-smelling chemical.
Thioacetone was discovered in 1889 as a minor impurity in the synthesis of trithioacetone by Baumann and Fromm.
Properties
Thioacetone is an organosulfur compound and has been isolated as an orange or brown liquid at low temperatures.
- Density: 0.9±0.1 g/cm3
- Boiling Point: 59.7±23.0 °C at 760 mmHg
- Vapour Pressure: 211.5±0.1 mmHg at 25°C
- Enthalpy of Vaporization: 29.0±3.0 kJ/mol
- Flash Point: -10.1±22.6 °C
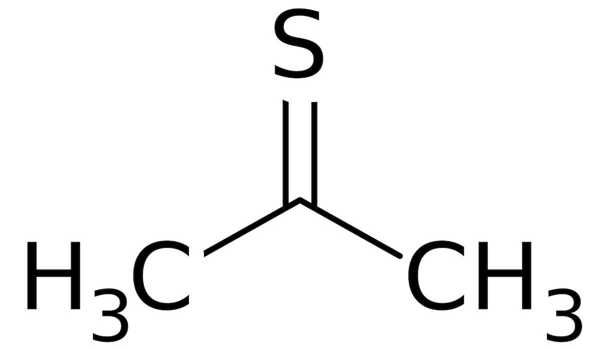
Preparation
Thioacetone is typically derived from the cyclic trimer trithioacetone, [(CH3)2CS]3. Pyrolysis of allyl isopropyl sulfide or treatment of acetone with hydrogen sulfide in the presence of a Lewis acid yields the trimer. To produce thione, the trimer cracks at 500–600°C (932–1,112 °F).
In 1889, a factory in Freiberg attempted to produce thioacetone. Thioacetone is difficult to produce — the compound (CH3)2CS remains liquid only at temperatures below -20 degrees Celsius; at higher temperatures, it clumps together and converts to a white solid known as trithioacentone — but it stinks horribly in either form.
Odor
Thioacetone has a very strong odor. The smell, like that of many low molecular weight organosulfur compounds, is potent and can be detected even when highly diluted. An attempt to distill the chemical in the German city of Freiburg in 1889 was met with cases of vomiting, nausea, and unconsciousness in a radius of 0.75 kilometers (0.47 mi) around the laboratory due to the odor.
In an 1890 report, British chemists at the Whitehall Soap Works in Leeds noted that dilution seemed to make the smell worse and described it as “fearful.” Thioacetone is classified as a hazardous chemical due to its extremely foul odor and ability to knock people out, induce vomiting, and be detected over long distances.