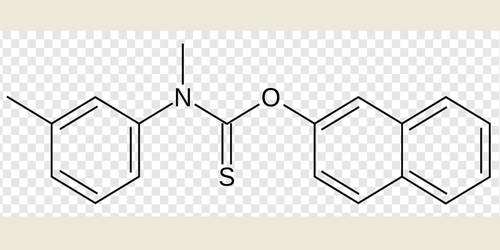
Thioketones (thiocarbonyls) are organosulfur compounds related to conventional ketones in which the oxygen has been replaced by a sulfur. They are generally readily available and undergo [4 + 2] cycloadditions under mild thermal conditions. Instead of a structure of R2C=O, thioketones have the structure R2C=S, which is reflected by the prefix “thio-” in the name of the functional group. The reaction of thioketones with any compound with an activated double C C bond leads to the formation of a thietane. Unhindered alkylthioketones typically tend to form polymers or rings.
C bond leads to the formation of a thietane. Unhindered alkylthioketones typically tend to form polymers or rings.
Structure and bonding
It is a compound that is a ketone in which oxygen is replaced by sulfur and which in general is readily polymerizable. The C=S bond length of thiobenzophenone is 1.63 Å, which is comparable to 1.64 Å, the C=S bond length of thioformaldehyde, measured in the gas phase. Due to steric interactions, the phenyl groups are not coplanar and the dihedral angle SC-CC is 36°. The synthesis of 4-alkyl thioketones is possible by exploiting the stabilizing effect of a sulfur atom upon an adjacent carbanionic center.
Consistent with the double bond rule, most alkyl thioketones are unstable with respect to dimerization. The energy difference between the p orbitals of sulfur and carbon is greater than that between oxygen and carbon in ketones. The relative difference in energy and diffusivity of the atomic orbitals of sulfur compared to carbon results in a poor overlap of the orbitals and the energy gap between the HOMO and LUMO is thus reduced for C=S relative to C=O. The striking blue appearance of thiobenzophenone is due to π→ π* transitions upon the absorption of light with a wavelength of 314.5 nm.
Preparative methods
Thiones are usually prepared from ketones using reagents that exchange S and O atoms. The most widely studied reactions have been those of thiourea and alkyl thioureas. A common reagent is phosphorus pentasulfide and the related reagent Lawesson’s reagent. Other methods use a mixture of hydrogen chloride combined with hydrogen sulfide. Bis(trimethylsilyl)sulfide has also been employed.
Thiobenzophenone [(C6H5)2CS] is a stable deep blue compound that dissolves readily in organic solvents. It photo oxidizes in air to benzophenone and sulfur. Since its discovery, a variety of related thiones have been prepared.