Titration is a common laboratory method of using quantitative chemical analysis. It is a technique to determine the concentration of an unknown solution. It is also known as titrimetry and volumetric analysis is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance to be analyzed). This method is used to determine the unidentified concentration of a known analyte.
A quantitative and volumetric technique, to determine the unknown concentration of a solution by the known concentration of a solution in the presence of an indicator is called Titration.
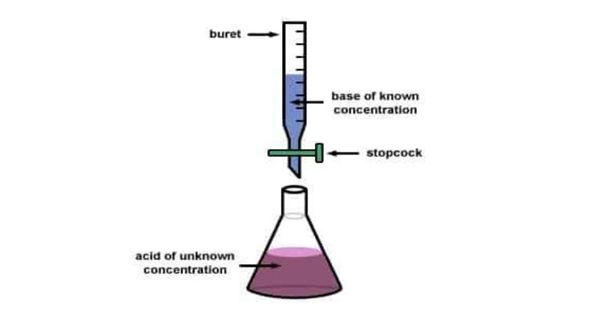
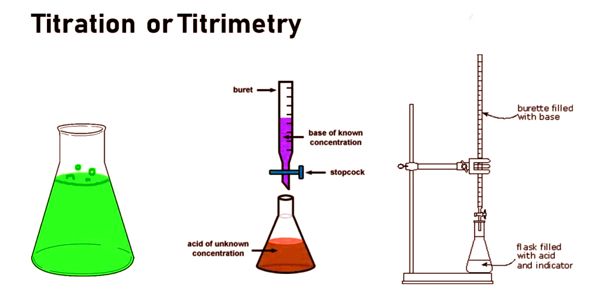
A reagent, termed the titrant or titrator, is prepared as a standard solution of known concentration and volume. The process is usually carried out by gradually adding a standard solution (i.e., a solution of known concentration) of titrating reagent, or titrant, from a burette, essentially a long graduated measuring tube with a stopcock and a delivery tube at its lower end. The titrant reacts with a solution of analyte (which may also be termed the titrand) to determine the analyte’s concentration. The volume of titrant that reacted with the analyte is termed the titration volume.
Procedure
Typically, the titrant (the know solution) is added from a buret to a known quantity of the analyte (the unknown solution) until the reaction is complete. A reagent, called the titrant or titrator, is prepared as a standard solution. A known concentration and volume of the titrant reacts with a solution of the analyte or titrand to determine concentration. A typical titration begins with a beaker or Erlenmeyer flask containing a very precise amount of the analyte and a small amount of indicator (such as phenolphthalein) placed underneath a calibrated burette or chemistry pipetting syringe containing the titrant. The volume of the titrant reacted is called the titration volume. There are many types of titrations with different procedures and goals. The most common types of qualitative titration are acid-base titrations and redox titrations. Knowing the volume of titrant added allows the determination of the concentration of the unknown. Often, an indicator is used to usually signal the end of the reaction, the endpoint.
Acid-base titrations depend on the neutralization between an acid and a base when mixed in solution. The acid-base indicator indicates the endpoint of the titration by changing color. Small volumes of the titrant are then added to the analyte and indicator until the indicator changes color in reaction to the titrant saturation threshold, representing arrival at the endpoint of the titration, meaning the amount of titrant balances the amount of analyte present, according to the reaction between the two.
Information Source: