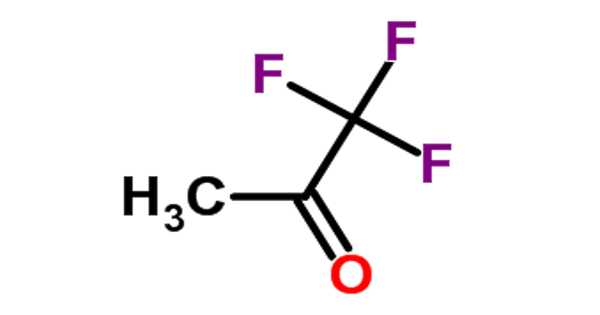
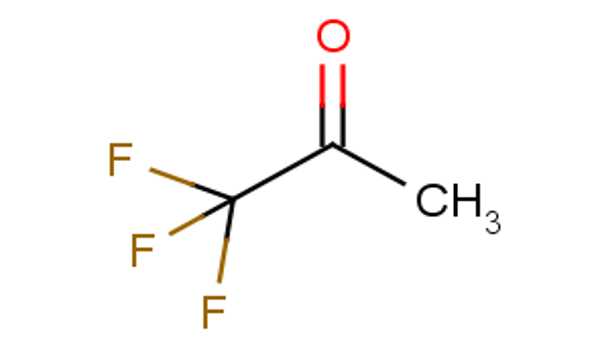
Trifluoroacetone [CF3C(O)CH3] is an organofluorine compound with the formula CF3C(O)CH3. The compound is a colorless liquid with an odor similar to chloroform.
Keep away from ignition sources and use explosion-proof equipment. Keep the container tightly closed in a well-ventilated, dry fume hood. It is advised to store at 2–8°C. Over time, pressure can build up, causing containers to burst.
Properties
Trifluoroacetone exhibits reactivities not normally seen in common ketones. Aside from influencing physical properties, the trifluoromethyl group increases the electrophilicity of the carbonyl center. As with formaldehyde, its hydration equilibrium is primarily on the side of the geminal diol in an aqueous solution.
- Melting point: -78 °C
- Boiling point: 22 °C(lit.)
- Density: 1.252 g/mL at 25 °C(lit.)
- Vapor pressure: 13.62 psi ( 20 °C)
- Flash point: −23 °F
- Storage temp.: 2-8°C
- Form: Liquid
- Color: Clear colorless
- Water Solubility: Miscible
Preparation and reactions
It can be synthesized in a variety of ways. The compound was first synthesized by decarboxylating ethyl 4,4,4-trifluoro-3-oxobutanoate (CF3COCH2CO2Et). It has been reported that by treating trifluoroacetic acid (CF3CO2H) with an excess of methylmagnesium iodide, 56 percent yield of -trifluoroacetone (CF3COCH3) can be obtained (MeMgI, 3 equiv).
CF3COCH3 was obtained in 86 percent yield through the reaction of TMSCF3 and ethyl acetate in the presence of cesium fluoride using the Ruppert–Prakash reagent (TMSCF3) (CsF). In addition to the methods described above, CF3COCH3 was obtained in a low yield via a Cu(I)-catalyzed reaction of sodium trifluoroacetate (CF3CO2Na) and acetyl chloride (CH3COCl).
Trifluoroacetone is produced by decarboxylation of trifluoroacetoacetic acid:
CF3C(O)CH2CO2H → CF3C(O)CH3 + CO2
The acetoacetic acid in turn is obtained via condensation of acetate and trifluoroacetate esters.
Trifluoroacetone has been examined as an oxidizing agent in Oppenauer oxidation, in which case hydroxyl groups of secondary alcohols can be oxidized in the presence of hydroxy groups of primary alcohols.
Uses
Starting with 2,6-dihalopyridines, trifluoracetone is used in the synthesis of 2-trifluoromethyl-7-azaindoles. By using a Strecker reaction followed by either nitrile hydrolysis or reduction, the derived chiral imine is used to prepare enantiopure -trifluoromethyl alanines and diamines.
Precautions
Keep away from ignition sources and use explosion-proof equipment. Keep the container tightly closed in a well-ventilated, dry fume hood. It is advised to store at 2–8°C. Over time, pressure can build up, causing containers to burst.