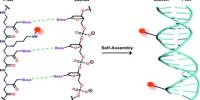
According to a team of researchers, the mechanism behind one of the first stages of a coal formation may not be what we thought it was. They discovered that microbes were responsible for coal formation and methane production in these areas, which has implications for methane fuel recovery from some coal fields.
The researchers examined methoxyl groups in coal samples from around the world and used stable isotopes to demonstrate that organic material eventually turns into coal due to microbial action. A methoxyl group is made up of a carbon atom, three hydrogen atoms, and an oxygen atom. The oxygen atom has the ability to attach to any number of sites in a larger molecule. In the case of coal, it binds to a carbon atom in one of the ring arrangements of coal.
“Most geochemists would say that coal was formed by temperature, acids, or catalysts,” said Max K. Lloyd, assistant research professor of geosciences at Penn State. “However, our findings contradict those mechanisms. They show that microbes consume coal methoxyl groups directly, transforming coal and producing methane.”
The challenge with coal bed methane production is that drilling wells is very expensive, and the well could run dry in a month. We have no idea why. Producers add more microbes or nutrition, but only if those are the limiting factors, not if the coal itself is the limiting factor.
Max K. Lloyd
When plant matter in wetland forests falls into the water and is quickly buried, coal forms. As the organic material is buried deeper and becomes more concentrated in carbon, it transforms from peat to lignite, then sub-bituminous, bituminous, and finally anthracite. Anthracite coal is mostly carbon, whereas lignite is still mostly planted matter.
According to Lloyd, the majority of coal used today in places like India and China is lignite or sub-bituminous because these are the only types that are easily and cheaply available, but when burned, these coals emit the most greenhouse gases. As a solution, methane wells in these coal beds – coal bed methane (CBM) – are appealing as a bridge away from fossil fuels, but “CBM production wells often have limited life spans,” the researchers write in Science.
“The challenge with coal bed methane production is that drilling wells are very expensive, and the well could run dry in a month,” Lloyd explained. “We have no idea why. Producers add more microbes or nutrition (for the microbes), but only if those are the limiting factors, not if the coal itself is the limiting factor.”

Lloyd was researching the abundance of methoxyl groups in living or recently dead trees when he met Elizabeth Trembath-Reichert, now an assistant professor in the University of Arizona’s School of Earth and Space Exploration, who was researching microbes that consume methyl groups in coal. Lloyd began looking for the same thing in coal from around the world after they confirmed the observations were real using two methods.
According to the researchers, methane is formed from methoxyl groups in coal, but how methane forms from coal are poorly understood. The researchers looked at stable carbon isotopes in the methoxyl groups left behind to better understand this process.
Stable isotopes are non-radioactive isotopes of an element with a variable number of neutrons in its nucleus. Carbon isotopes with 12 and 13 neutrons are nearly identical, with the exception that carbon 13, while less abundant in nature, is slightly heavier. Because biological organisms prefer one isotope over the other, what remains in the original source will differ from the percentages of the isotopes normally found.
When Lloyd and his colleagues examined methoxyl groups in everything from wood to bituminous coal, they discovered that the isotope profile did not match what would be expected if methane was formed through heat, acidity, or catalytic reactions, but it did match the patterns expected from microbial action.
“It turns out that aerobic microbes are great at degrading the rings in coal, but anaerobic microbes have no good way of disassembling the rings,” Lloyd explained. “One of the only things the anaerobes have left to do is cut off the methoxyl portions.”
The methoxyl groups that have been liberated are then converted to methane. However, once all of the available methoxyl radicals have been sheared from the rings, the microbes are unable to access anything else, and the reaction comes to a halt and the well runs dry.
“What’s really fascinating is that these microbes are releasing enzymes to cut off the methoxyl,” Lloyd explained. “They’re degrading the structure extracellularly, which is limiting because coal isn’t a solution and the microbe can’t get everywhere in the coal structure.”
The researchers believe that the depletion of methoxyl groups in coal over time indicates that the coal itself is the limiting factor in methane production. As a result, adding more microbes or nutrients will not result in more methane production, and another approach is required.