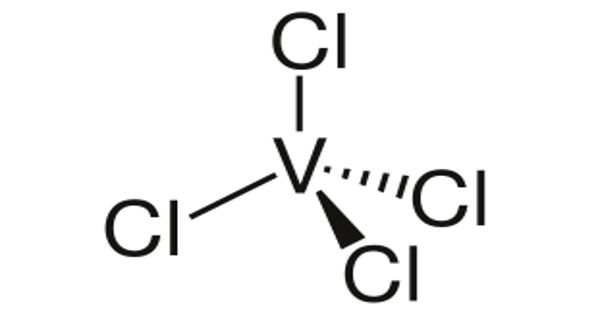
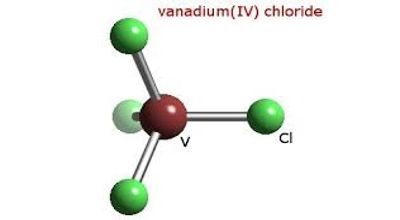
Vanadium tetrachloride is an excellent water-soluble crystalline Vanadium source for uses compatible with chlorides. It is the inorganic compound with the formula VCl4. Chloride compounds can conduct electricity when fused or dissolved in water. Chloride materials can be decomposed by electrolysis to chlorine gas and the metal. This bright red liquid serves as a useful reagent for the preparation of other vanadium compounds. They are formed through various chlorination processes whereby at least one chlorine anion (Cl-) is covalently bonded to the relevant metal or cation.
Vanadium tetrachloride is prepared by chlorination of vanadium metal. It is a catalyst for the polymerization of alkenes, especially those useful in the rubber industry.
Synthesis, bonding, basic properties
Vanadium is a bright white, soft, ductile metal with good structural strength. With one more valence electron than diamagnetic TiCl4, VCl4 is a paramagnetic liquid. Vanadium is resistant to attack by alkalis, hydrochloric acid, sulfuric acid, and saltwater. It is one of only a few paramagnetic compounds that are liquid at room temperature.
- Molecular Formula: Cl4V
- Molecular Weight (g/mol): 192.75
- Formula Weight: 192.75
- Physical Form: Liquid
- Percent Purity: 99+%
- Density: 1.8200g/mL
- Melting Point: -28 °C (-18.4 °F)
- Boiling Point: 154° C (309.2° F)
Vanadium is a comparatively abundant element that enjoys a plethora of biological and industrial applications and does also impact environmental aspects both in the context of problematic issues and beneficial outcomes, such as rendering dinitrogen available for plant growth and development. VCl4 is prepared by chlorination of vanadium metal. VCl5 does not form in this reaction; Cl2 lacks the oxidizing power to attack VCl4. VCl5 can however be prepared indirectly from VF5 at −78°C. In contrast, the heavier analogs NbCl5 and TaCl5 are stable and not particularly oxidizing. VF5 can be prepared directly by fluorination of vanadium metal, reflecting the increased oxidizing power of F2 vs Cl2. Indicative of its oxidizing power, VCl4 releases Cl2 at its boiling point (standard pressure) to afford VCl3. Vanadium metal is obtained by reducing vanadium(V) oxide with calcium in a pressure vessel. Vanadium of high purity can be obtained by reducing vanadium(III) chloride with magnesium.
Applications
VCl4 is a catalyst for the polymerization of alkenes, especially those useful in the rubber industry. About 80% of the vanadium produced is used as a steel additive. Vanadium-steel alloys are very tough and are used for armour plates, axles, tools, piston rods, and crankshafts. The underlying technology is related to Ziegler–Natta catalysis, which involves the intermediacy of vanadium alkyls. It is used as a pigment for ceramics and glass, as a catalyst, and in producing superconducting magnets.