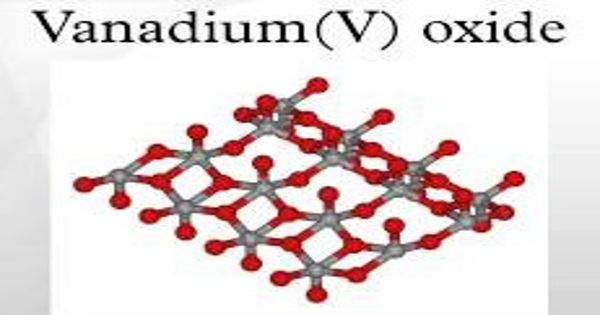
Vanadium (V) oxide is a highly insoluble thermally stable Vanadium source suitable for glass, optic, and ceramic applications. It is also known as vanadia, is the inorganic compound with the formula V2O5. It appears as a yellow to red crystalline powder. Commonly known as vanadium pentoxide, it is a brown/yellow solid, although when freshly precipitated from an aqueous solution, its color is deep orange. Contact may cause severe irritation to the skin, eyes, and mucous membranes. Because of its high oxidation state, it is both an amphoteric oxide and an oxidizing agent. May be toxic by ingestion, inhalation, and skin absorption.
Vanadium (V) oxide is a brown/yellow solid, although when freshly precipitated from an aqueous solution, its color is deep orange. It is a poisonous brown/ yellow solid that is the most stable and abundant compound of vanadium.
Vanadium takes its name from the Scandinavian goddess Vanadis and was discovered in 1801 by Andrés Manuel del Rio. It was isolated in 1867 by Henry Roscoe as a silvery-white metal that is somewhat heavier than aluminum but lighter than iron. Vanadium oxides have been a subject of intense study in lithium metal negative electrode rechargeable cells since 1975.
Properties
- Compound Formula: O5V2
- Molecular Weight: 181.88
- Appearance: Yellow to rust color
- Melting Point: 690 °C
- Boiling Point: 1750 °C
- Density: 3.35 g/cm3
- Solubility in H2O: N/A
From the industrial perspective, it is the most important compound of vanadium, being the principal precursor to alloys of vanadium, and is a widely used industrial catalyst. They are relevant to a multitude of catalytic reactions. They are compounds containing at least one oxygen anion and one metallic cation. Slightly soluble in water and denser than water.
Vanadium oxides, a prominent class of the early 3d transition metal compounds, have been objects of great interest in the past few decades due to their very broad range of oxidation states.
Vanadium oxide is a poisonous brown/ yellow solid that is the most stable and abundant compound of vanadium. The mineral form of this compound, shcherbinaite, is extremely rare, almost always found among fumaroles. A mineral trihydrate, V2O5·3H2O, is also known under the name of navajoite. Vanadium Oxide is also available in pellets, pieces, powder, sputtering targets, tablets, and nanopowder.