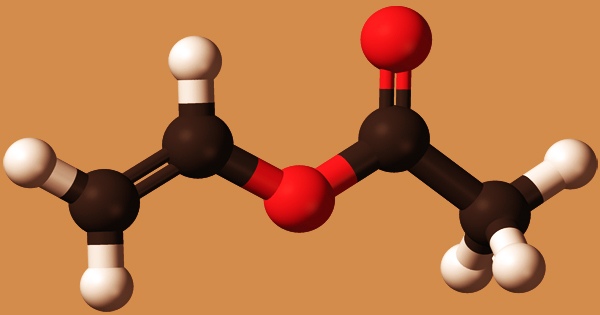
Vinyl acetate is a colorless, transparent liquid, and it is an organic chemical with the formula CH3CO2CH=CH2. Polyvinyl acetate, an essential commercial polymer, is made from this colorless liquid. In tiny amounts, it has a pleasant, fruity aroma, but at greater concentrations, it has a harsh, stinging odor. Flashpoint 18°F; density 7.8 lb/gal, and slightly soluble in water. Vapors have a higher density than air. The eyes and respiratory system are irritated by vapors. If heated or polluted, it may polymerize. The container may violently rupture if the polymerization occurs inside it.
Vinyl acetate is a chemical building component that may be found in a wide range of industrial and consumer goods. In 2007, the global capacity for vinyl acetate production was projected to be 6,969,000 tonnes per year, with the majority of capacity located in the United States (1,585,000 tonnes, all in Texas), China (1,261,000 tonnes), Japan (725,000 tonnes), and Taiwan (650,000). Paints, adhesives, coatings, textiles, wire, and cable polyethylene compounds, laminated safety glass, packaging, automobile plastic fuel tanks, and acrylic fibers all employ emulsion polymers, resins, and intermediates.
Vinyl acetate is a chemical that is widely used in industry in the United States. In 2008, the average list price per tonne was $1600. Celanese is the major producer (about 25% of global capacity), while China Petrochemical Corporation (7%), Chang Chun Group (6%), and LyondellBasell (5%) are other important producers. Polyvinyl acetate emulsions and resins are made from it. In products made with vinyl acetate, such as molded plastic objects, adhesives, paints, food packing containers, and hairspray, very tiny residual amounts of vinyl acetate have been discovered.
Heat, sparks, or flames may all ignite vinyl acetate, making it very flammable. It’s utilized in the production of various industrial compounds. It’s the vinyl alcohol acetate ester. Vinyl acetate is more difficult to make than other acetate esters because vinyl alcohol is very unstable (in comparison to acetaldehyde). The reaction of ethylene and acetic acid with oxygen in the presence of a palladium catalyst is the most used industrial method.
2 C2H4 + 2 CH3CO2H + O2 → 2 CH3CO2CHCH2 + 2 H2O
Vinyl acetate passes through many of the same reactions as an alkene and an ester. Isotope labeling and kinetics studies indicate that PdCH2CH2OAc-containing intermediates are involved in the process. Eliminating beta-hydride would result in vinyl acetate and palladium hydride, which would then be oxidized to produce hydroxide. Bromine is added to make dibromide. Because the matching halo-alcohols are not available, hydrogen halides are added to give 1-haloethyl acetates, which cannot be produced by conventional techniques.
These compounds are mostly utilized in the packaging and construction sectors to produce glues. Paints, fabrics, and paper are all made with them. Vinyl acetate is also utilized as a food starch modification and as a coating in plastic sheets for food packaging. In the presence of palladium catalysts, acetic acid reacts to form ethylidene diacetate, CH3CH(OAc)2. A number of carboxylic acids are used to transesterify it. Diels-Alder and 2 + 2 cycloadditions are also performed on the alkene.
Hydroesterification was formerly used to make vinyl acetate. The gas-phase addition of acetic acid to acetylene in the presence of metal catalysts is used in this technique. Organic and inorganic peroxides, azo compounds, redox systems, which may contain organometallic components, light, and high-energy radiation can all begin polymerization. It may be used to make ethylene-vinyl acetate (EVA), vinyl acetate-acrylic acid (VA/AA), polyvinyl chloride acetate (PVCA), and polyvinylpyrrolidone (Vp/Va Copolymer, used in hair gels) copolymers with other monomers. The container may violently rupture if the polymerization occurs inside it.
Because of the radical’s fragility, most “living/controlled” radical methods have had difficulty controlling polymerization. It’s possible that dangerous polymerization will develop. An inhibitor is usually present to prevent self-polymerization. Polymerization can be triggered by high temperatures or oxidation. The addition of a xanthate or a dithiocarbamate chain transfer agent to RAFT (or more precisely, MADIX) polymerization provides a simple means of regulating the production of polyvinyl acetate (PVA).
Vinyl acetate has been linked to problems with reproduction. It’s an irritant to the skin and upper respiratory tract, as well as a depressive to the central nervous system. The cardiac muscles gradually deteriorated as a result of the exposure. Several major industrial explosions have occurred as a result of reactions with air or water to produce peroxides that accelerate an exothermic polymerization process. Large industrial explosions have occurred as a result of solution polymerization of acetate dissolved in toluene.
Vinyl acetate burns and produces noxious odors when heated to breakdown. When exposed to heat, flames, or oxidizers, it is extremely hazardous; strong acids and oxidizers offer an explosive risk. Vinyl acetate may be transesterified using an iridium catalyst, resulting in vinyl ethers:
ROH + CH2=CHOAc → ROCH=CH2 + HOAc
Polymers are used to make adhesives, paints, paper coatings, and textile finishes. They are generally produced as emulsions, suspensions, solutions, or resins. Vinyl acetate with a low molecular weight is utilized as a chewing gum basis. After inhalation, vinyl acetate tended to stay in the body; 70% of the vinyl acetate given was retained, and equilibrium was reached during the first few minutes of exposure. Vinyl acetate has low toxicity, according to tests.
Information Sources: