A zeotropic mixture is a concoction of liquid components with varying boiling points. It is a mixture of components with varying boiling points. Nitrogen, methane, ethane, propane, and isobutane, for example, form a zeotropic mixture. Individual constituents of a mixture do not evaporate or condense at the same temperature as one another. We can call it a non-azeotropic mixture because it is the inverse of an azeotropic mixture.
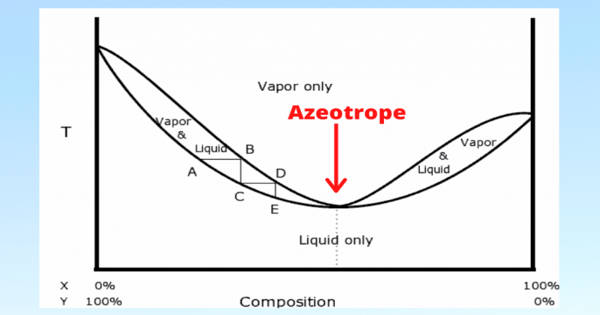
In other words, the mixture has a temperature glide because the phase change occurs in a temperature range of four to seven degrees Celsius rather than at a constant temperature. Individual components do not evaporate or condense at the same temperature due to differences in boiling points. This temperature glide can be seen on temperature-composition graphs as the temperature difference between the bubble point and the dew point. As a result, the mixture is gliding in temperature.
For zeotropic mixtures, the temperatures on the bubble (boiling) curve are between the individual component’s boiling temperatures. The phase changes of the liquid components take place in a series of temperatures rather than at the same temperature. When a zeotropic mixture is boiled or condensed, the composition of the liquid and the vapor changes according to the mixtures’ temperature-composition diagram.
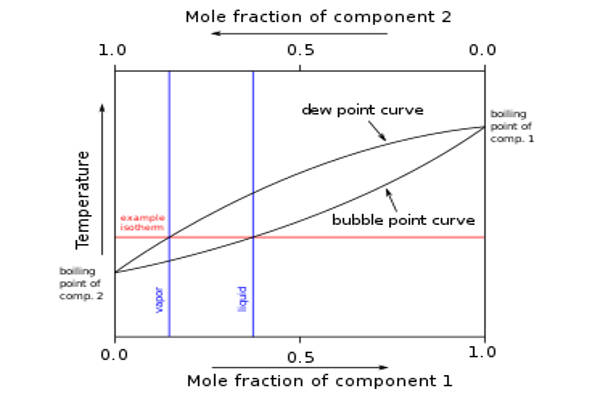
We can see the boiling temperatures of the components between bubble point and dew point if we draw a temperature vs. composition graph for a zeotropic mixture. The temperature at which the first bubble of vapor forms is known as the bubble point. The temperature at which condensation occurs is referred to as the dew point. The temperature vs. composition graph depicts how the composition of liquid and vapor changes during boiling, condensing, and intermediate temperatures. A zeotropic mixture is one that contains ethane, methane, nitrogen, propane, and isobutene.
In nucleate and convective boiling, as well as the organic Rankine cycle, zeotropic mixtures exhibit distinct properties. Because zeotropic mixtures have different properties than pure fluids or azeotropic mixtures, they have a wide range of applications in industry, including distillation, refrigeration, and cleaning processes.
Azeotropic mixtures have pressure maximums/temperature minimums or pressure minimums/temperature maximums that are greater than/less than the values of the pure components. For some commercially available blends, stating the compositions of zeotropic mixtures is common, but the term zeotropic is only valid for a given temperature and pressure, not for specific compositions.
Informtion Source: