Using an RNA-based medication that was given to the cells by specific lipid nanoparticles, Tel Aviv University researchers eliminated 60% of multiple myeloma blood cancer cells in human tissues obtained from patients at Rabin Medical Center (Belinson Hospital) and 90% of multiple myeloma blood cancer cells in laboratory settings.
The researchers developed lipid-based nanoparticles (similar to those used in the COVID-19 vaccine) containing RNA molecules that silence the gene CKAP5, encoding cytoskeleton-associated protein 5.
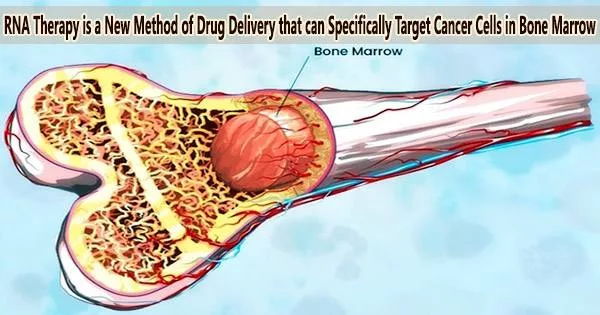
Because the cancer cell cannot divide when this protein is blocked, it is effectively killed. The nanoparticles were coated with antibodies that directed them exclusively to the cancer cells inside the bone marrow, preventing them from harming non-cancerous cells.
The breakthrough was achieved by a group of researchers from Tel Aviv University and the Rabin Medical Center, led by Prof. Dan Peer, a pioneer in the development of RNA therapeutics and Head of the Nanomedicine Laboratory at the Shmunis School of Biomedicine and Cancer Research, also serving as TAU’s VP R&D, and by Ph.D. student Dana Tarab-Ravski. The results were published in the journal Advanced Science.
Dana Tarab-Ravski explains, “Multiple myeloma is a blood cancer usually found in the older population. While most blood cancers appear in the blood stream or lymph nodes and spread from there to the rest of the body, multiple myeloma cells appear and form tumors inside the bone marrow and are therefore very hard to reach.”
Today RNA therapeutics are approved for treating a genetic liver disease and for vaccines injected into the muscle, as we saw with the COVID-19 vaccines. The drug delivery system we developed is the first that specifically targets cancer cells inside the bone marrow, and the first to show that silencing the expression of CKAP5 gene can be used to kill blood cancer cells.
Dana Tarab-Ravski
The study’s results are highly positive: in flasks of cells generated in the lab, almost 90% of the cancer cells were eliminated by the nanoparticles created by the researchers.
At the Rabin Medical Center’s haemato-oncological ward, cancer samples obtained from multiple myeloma patients were used to evaluate the new therapy in its second stage. The success rate in these samples was 60%.
The researchers used an animal model to test the nanoparticles’ ability to enter the bone marrow, and they discovered that after just one injection, the RNA had reached 60% of the multiple myeloma cancer cells there.
In the animal model used to test the nanoparticles’ therapeutic efficacy, two-thirds of the cancer cells were eliminated, and the animals displayed notable improvements in all clinical indications.
“People with multiple myeloma suffer from severe pain in their bones, as well as anemia, kidney failure, and a weakened immune system,” says Tarab-Ravski. “There are many possible treatments for this disease, but after a certain period of improvement most patients develop resistance to the therapy and the disease relapses even more aggressively. Therefore, there is a constant need for developing new treatments for multiple myeloma.”
“RNA-based therapy has a great advantage in this case because it can be developed very quickly. By simply changing the RNA molecule a different gene can be silenced each time, thereby tailoring the treatment to the progression of the disease and to the individual patient. The challenge in these treatments is to reach the right cells.”
“Today RNA therapeutics are approved for treating a genetic liver disease and for vaccines injected into the muscle, as we saw with the COVID-19 vaccines. The drug delivery system we developed is the first that specifically targets cancer cells inside the bone marrow, and the first to show that silencing the expression of CKAP5 gene can be used to kill blood cancer cells.”
Peer states, “Our technology opens a new world for selective delivery of RNA medications and vaccines for cancer tumors and diseases originating in the bone marrow.”