The initiation of routine colonoscopies, during which an endoscope fitted with a light and a camera is used to visually inspect the colon for signs of cancer, begins when a person in the United States turns 45.
Colorectal cancer is relatively slow-growing and, if discovered early, is frequently treatable surgically. The fourth biggest cause of cancer-related deaths in the nation, it is more challenging to cure the longer it goes untreated.
Despite the availability of this highly visual screening approach, traditional histology pathologists still primarily use slides of tumor samples to analyze colorectal cancer in order to make treatment decisions for specific patients.
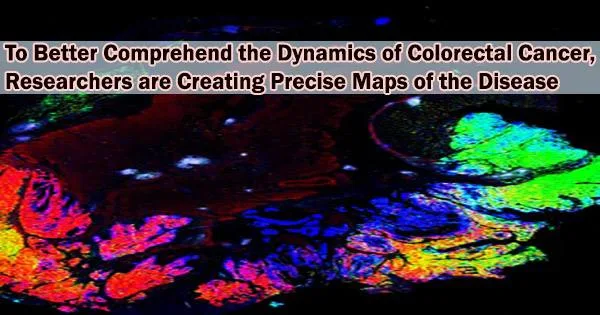
Currently, a team from Harvard Medical School has created massive 2D and 3D spatial maps of colorectal cancer by fusing histology with cutting-edge single-cell imaging technology. The maps, which are presented in Cell, combine a wealth of genomic data with histological features to provide new details about the cancer’s structure, development, and interactions with the immune system.
“Our approach provides a molecular window into 150 years of diagnostic pathology and reveals that many of the elements and structures traditionally thought to be isolated are actually interconnected in unexpected ways,” said co-senior author Peter Sorger, the Otto Krayer Professor of Systems Pharmacology in the Blavatnik Institute at HMS. “An analogy is that before we were just looking at the tail or the foot of the elephant, but now, for the first time, we can start to see the whole elephant at once.”
The atlases will be made available to the scientific community for free as part of the National Cancer Institute’s Human Tumor Atlas Network. The maps are a part of the team’s larger efforts to produce atlases for other cancer kinds.
In-depth maps of early-stage melanoma were previously produced by the researchers using a similar methodology, and maps for other malignancies are also being developed. The team’s ultimate goal is for these cancer atlases to advance research and enhance detection and therapy.
Combining old and new
Histology has long been the cornerstone of cancer diagnosis and treatment: Pathologists examine a tumor sample stained with hematoxylin and eosin (H&E) under a microscope and pick out key features to determine the grade and stage of the cancer. Oncologists to develop a treatment plan, which usually involves some combination of surgery, drugs, and radiation, use this information. H&E-based histology is relatively simple, cheap, fast, and can reveal a lot about a tumor.
Our approach provides a molecular window into 150 years of diagnostic pathology and reveals that many of the elements and structures traditionally thought to be isolated are actually interconnected in unexpected ways. An analogy is that before we were just looking at the tail or the foot of the elephant, but now, for the first time, we can start to see the whole elephant at once.
Professor Peter Sorger
“Our existing maps of colorectal cancer originate in pathology over the course of 150 years, we’ve figured out the most important H&E features for diagnosing a patient,” said co-senior author Sandro Santagata, HMS associate professor of systems biology and associate professor of pathology at Brigham and Women’s Hospital.
Traditional histology has limitations, though, in that it can’t fully exploit the knowledge that has been learned about cancer over the past 50 years because it doesn’t capture the molecular structure or physical characteristics of a tumour.
“Histology is extremely powerful, but we often don’t know what it means in modern molecular terms,” Sorger said.
In the new paper, the researchers combined histology with single-cell molecular imaging data acquired through a multiplexed imaging technique called cyclic immunofluorescence, or CyCIF. They used this data to produce intricate 2D maps showing extensive colorectal cancer hotspots. Jia-Ren Lin, the first author, is the platform director in the Laboratory of Systems Pharmacology at HMS. He oversaw an initiative to combine these maps to create a sizable 3D reconstruction of a tumor.
“Our maps include information on almost 100 million cells from large pieces of tumors, and provide a rather unprecedented look at colorectal cancer,” Santagata said. They allow researchers to start asking key questions about differences between normal and tumor tissues and variation within a tumor, he added, and reveal “exciting architectural features that had never been observed before, as well as molecular changes associated with these features.”
The maps revealed that a single tumor can have areas that are more or less invasive and areas that look more or less malignant, leading to histological and molecular gradients where one part of a tumor changes into another.
“Within each tumor, there is a wide range of properties of colorectal cancer we see many different regions and neighborhoods that have distinct characteristics, as well as the transitions between them,” Santagata said. From here, he added, scientists can now explore what drives these differences within individual tumors.
For example, the maps showed that immune environments varied dramatically within a single tumor.
“They were as different across a single tumor as among tumors which is important because tumor-immune interactions are what you are trying to target with immunotherapy,” Sorger said.
The researchers saw that the T cells responsible for combating the cancer were not directly repressed by tumor cells but rather by other immune cells in the milieu surrounding the tumor, which is similar to their observation in melanoma.
“This gives us a whole new appreciation for how diverse and plastic the tumor environments are they are rich communities, and we are now better equipped to figure out how they develop,” Santagata said.
The maps also provided new insights into the architecture of the tumors. For example, scientists had previously identified what they thought were 2D pools of a mucus-like substance called mucin with clusters of cancer cells floating inside. However, in the new study, the 3D reconstruction revealed that these mucin pools are, in fact, a series of caverns interconnected by channels, with finger-like projections of cancer cells.
“It’s a wild, new look at these tumor structures that we never really appreciated before,” Santagata said. “Because we can see them in 3D, we have a crisp, clean view of the structures, and we can now study why they are there, how they form, and how they shape tumor evolution.”
Translating results
The ultimate objective of these colorectal cancer maps, as well as all of the cancer atlases the team is creating, is to advance knowledge and enhance detection and therapy. Sorger stated that although precision medicine is a crucial component of treatment and entails adjusting medication to a patient’s specific malignancy, it is limited by pathology and genetics alone.
“The big translational story here is building the knowledge to make precision medicine practical for most patients,” he said. “We are currently working with Brigham and Women’s and the Dana-Farber Cancer Institute to determine how our methods can be used in a clinical setting.”
“This is allowing us to extract a whole additional layer of molecular and structural features that we think will provide diagnostic and prognostic information and improve our ability to target these cancers,” Santagata added.
The team now wants to continue incorporating new imaging technologies into their maps and improve their capacity to produce 3D reconstructions of tumors. Additionally, they intend to expand the cohort of colorectal cancer samples they are using for mapping and investigate the fundamental biology of the disease as shown by their maps.
For Sorger, the project represents an unusual collaboration between pathologists, engineers, and computational scientists: The pathologists identified important traits to be analyzed using machine learning as the imaging data poured in, and the computational scientists utilized machine learning to identify interesting findings that they presented to the pathologists.
“This was an extraordinarily close conversation between the computational group and the pathology group, going back and forth between the rich history of medicine known to pathologists and modern machine learning methods.” Sorger said. “I think it’s an exciting glimpse of how these computation methods can be used in medicine in the future, wherein you tightly couple biologists and physicians with computation, rather than seeing them as replacements for each other.”
The group started with melanoma and colorectal cancer since they are frequent cancers with unmet medical needs, large, solid tumors, and significant therapy options.
Next, the researchers plan to tackle breast cancer and brain cancer. To enable the development of further atlases, they also intend to teach other researchers in the use of imaging technology to create their own cancer maps.
“A new era in molecular pathology is beginning, and this is a deep look at a tumor that is showing us how remarkable the findings can be,” Santagata said.
Additional authors include Shu Wang, Yu-An Chen, Clarence Yapp, Madison Tyler, and Maulik Nariya of HMS; Shannon Coy of HMS and Brigham and Women’s; and Cody Heiser and Ken Lau of Vanderbilt University School of Medicine.