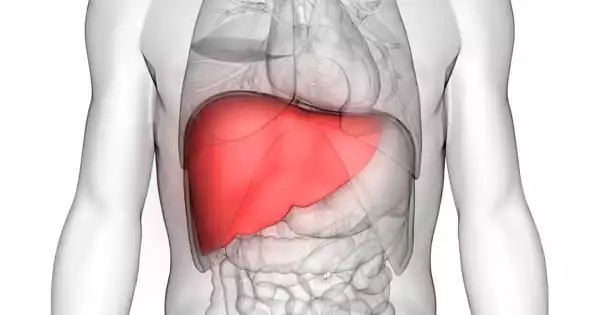
Mutations that link the liver disease to obesity and diabetes have been discovered, providing new insight into how systemic diseases interact. DNA mutations in liver cells that affect metabolism and insulin sensitivity in patients with liver disease have been identified for the first time. These mutations are only found in liver disease, which is linked to obesity, type 2 diabetes, and chronic alcohol consumption.
The Wellcome Sanger Institute, Cancer Research UK Cambridge Institute, Cancer Grand Challenges Mutographs team, and collaborators identified five genes that are mutated in people with liver disease and provided a better understanding of the role that three of these play in the disordered fat metabolism seen in non-alcoholic fatty liver disease (NAFLD) and chronic alcohol consumption.
The study, published in Nature, demonstrates that these mutations reduce the sensitivity of liver cells to insulin, with insulin resistance being a hallmark of type 2 diabetes. These findings show that mutations acquired during one’s lifetime can impair the liver’s ability to respond normally to dietary sugars and fats.
We identified five genes that are mutated in people with liver disease and provided a better understanding of the role that three of these play in the disordered fat metabolism seen in non-alcoholic fatty liver disease (NAFLD) and chronic alcohol consumption.
Dr. Stanley Ng
Understanding the pattern of genetic mutations in a patient’s liver may help identify the correct diagnosis in the future. These mutation patterns could also be used to characterize different subtypes of liver disease, potentially aiding in the matching of treatments to each group. While more research is needed, this research could lead to a new model for understanding how mutations in specific cell types can contribute to systemic metabolic diseases like diabetes.
It is currently estimated that there are approximately 1.5 billion cases of chronic liver disease worldwide, with the liver disease being the third leading cause of premature death in the United Kingdom. Chronic alcohol consumption, viral hepatitis, and NAFLD, which are linked to obesity and type 2 diabetes, are the most common causes of chronic liver disease.
This new study examined 1590 genomes from 34 patient liver samples, including healthy and diseased livers. The researchers discovered five genes in liver cells, also known as hepatocytes, that are mutated in patients with liver disease. Three of these genes are directly involved in how liver cells metabolize fat and respond to insulin.
When a large amount of alcohol or calories is consumed, insulin sends a signal to the liver cells to take up, process, and store a large amount of fat. If this continues for an extended period of time, the burden of storing this extra fat damages the cells, resulting in inflammation, chronic liver disease, and eventually scarring of the liver.
Cells with mutations in the genes identified in the paper do not respond to insulin signaling and thus do not absorb fat. This allows them to avoid the damage caused by fat storage and allows these mutated cells to survive and grow. However, while these mutations benefit the individual liver cell, they may impair that cell’s ability to contribute to the overall function of the liver.
Surprisingly, many of the patients were found to have multiple independent mutations in metabolism genes. This resulted in mutations affecting up to 15% to 25% of the entire liver in some patients, and having such a high number of liver cells carrying mutations could lead to organ-wide changes in liver function.
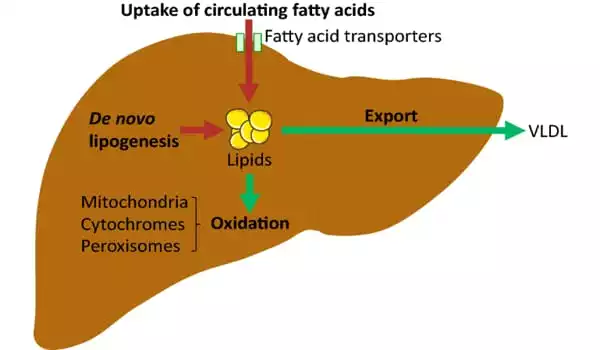
The same metabolism gene was frequently mutated within a single patient’s liver. However, the pattern of mutations differed between patients, implying that it may be possible to divide liver diseases into different subgroups based on their mutation patterns. It may be possible, with additional research, to develop and match novel treatments to these subgroups.
According to Dr. Stanley Ng, first author and Postdoctoral Fellow at the Wellcome Sanger Institute: “Liver disease is a complex condition that frequently coexists with other issues and conditions such as obesity and type 2 diabetes. However, the connection between these diseases is not well understood. While more research is needed to understand the genetic links between these conditions and the clinical implications of the mutations for our patients, our findings provide a fascinating new insight into systemic diseases and how to diagnose, manage, and treat them.”
“Understanding the role of these, and other, mutations in liver disease could help identify those who will be at higher risk of future complications, such as metabolic issues or liver cancer,” said Dr. Matthew Hoare, senior author, Advanced Clinician Scientist at the Cancer Research UK Cambridge Institute and member of the CRUK Cambridge Centre Early Detection Programme.
Interestingly, none of the mutations in metabolism genes were linked to the development of liver cancer, possibly because cancer cells are hungry for nutrients and these mutations may interfere with the cells’ ability to meet those metabolic demands. This information may be useful in understanding the changes that a liver cancer goes through as it develops from a background of chronic liver disease.”
Cancer Research UK’s Director of Cancer Grand Challenges, Dr. David Scott, stated: “The Cancer Grand Challenges Mutographs team is contributing to a better understanding of the relationship between mutations and cancer. This study demonstrates that the scope of that work extends beyond cancer, assisting us in learning more about the role of mutations in other diseases such as liver disease.”
Mutations acquired in specific cell types, such as liver cells, were not previously suspected of playing a role in the biology of conditions such as obesity and type 2 diabetes. This is the joy of science: we began this study hoping to understand how liver cancer develops from chronic liver disease, but we ended up proposing an exciting new model in which the same genetic event occurs many times independently within the liver, accounting for a significant fraction of liver cells collectively. The mutations may protect liver cells from toxicity, but only by allowing those cells to avoid their metabolic responsibilities.