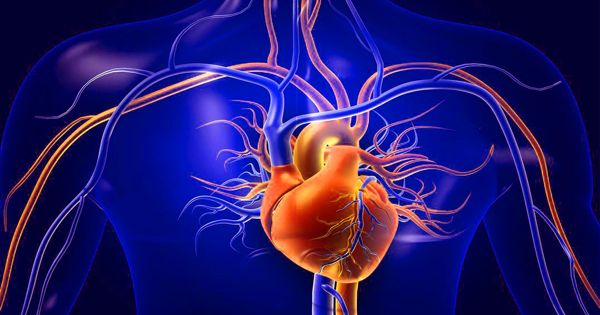
Researchers developed a mouse model of restrictive cardiomyopathy, a disease in which the heart muscle stiffens and the heart is unable to fill with blood properly. Their findings indicate that the disease is caused by an accumulation of mutant BAG3 protein, which interferes with the protein quality control system and the machinery for breaking down and recycling damaged proteins, causing disruptions in the heart muscle components.
Severe childhood restrictive cardiomyopathy is a condition that causes the muscles in the heart’s walls to stiffen, preventing the heart from filling properly with blood. A BAG3 protein mutation is known to cause restrictive cardiomyopathy, muscle weakness, difficulty taking in enough oxygen, and damage to multiple peripheral nerves, often significantly shortening the patient’s lifespan. There has yet to be a successful model for the disease, making research extremely difficult.
Researchers from the University of Tsukuba, Japan, collaborating with scientists from Germany, develop a mouse model for restrictive cardiomyopathy and uncover the underlying mechanisms of the disease.
However, researchers in Japan and Germany have developed a mouse model that mimics human pathology, making it possible to study the disease more easily. According to the findings, the restrictive cardiomyopathy caused by the BAG3 mutation alters the process by which damaged proteins are broken down and removed. Proteins accumulate in the cells, causing the cardiac muscle to malfunction.
The researchers were successful in expressing a human version of the mutant BAG3 protein in mouse cardiomyocytes, which are the cells that make up the heart muscle. “Our mouse model successfully mimicked the human disease,” says Assistant Professor Kenichi Kimura, the study’s lead author. “The mice developed increasingly severe symptoms of heart failure and growth retardation soon after birth, and they only lived for about five weeks.”
Cardiovascular diseases, the most common noncommunicable disease in the world, are responsible for a high mortality rate and a significant medical burden in countries worldwide, particularly in low- and middle-income countries. Because of their effective simulation of human cardiovascular diseases, strong reproductive ability, and ease of detection, experimental rodent models are widely used in cardiovascular disease research.
The researchers examined the heart tissue of mice expressing the mutant human BAG3 protein and discovered changes to the protein quality control system, which ensures proteins are properly folded, as well as increased levels of autophagy, a process by which damaged cells are removed and recycled. BAG3 participates in the breakdown of proteins that have been damaged by mechanical stress. The restrictive cardiomyopathy mutation involves a single base change in the DNA, resulting in a leucine amino acid in the mutant BAG3 protein where there should be a proline.
The team demonstrated that this causes the mutant protein to have reduced solubility and mobility, causing it to accumulate in muscle cells. This causes fibrosis, or scarring, and causes the heart muscle to stiffen and lose its ability to fully relax, preventing the heart from properly filling with blood. Furthermore, preliminary studies using a technique to knockdown and reduce mutant protein expression revealed that the researchers were able to alleviate disease symptoms in a mouse model.
Genetic cardiovascular disease in children is sometimes discovered during a crisis — a sudden collapse, difficulty breathing, or death in the family. Or it could be part of a broader set of symptoms that have nothing to do with the heart. It is sometimes picked up by chance.
Childhood restrictive cardiomyopathy is a rare but life-threatening condition. Hopefully, the information provided in this study, as well as the development of a mouse model of the disease to aid future research, will lead to the development of better treatments for children with this condition.