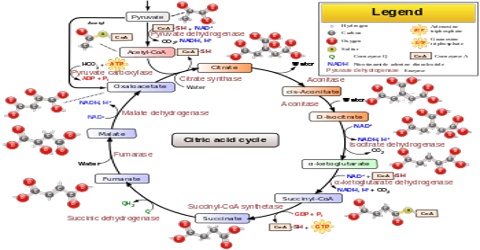
Citric Acid Cycle
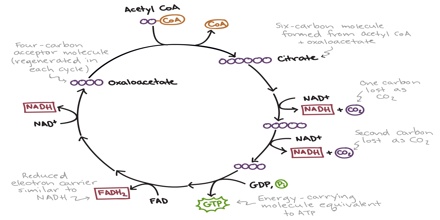
The citric acid cycle, also known as the Krebs cycle or the tricarboxylic acid cycle, is at the center of cellular metabolism, playing a starring role in both the process of energy production and biosynthesis. It finishes the sugar-breaking job started in glycolysis and fuels the production of ATP in the process. It is also a central hub in biosynthetic reactions, providing intermediates that are used to build amino acids and other molecules. The citric acid cycle enzymes are found in all cells that use oxygen, and even in some cells that don’t. The examples included here are taken from several different organisms.
In eukaryotic cells, the citric acid cycle occurs in the matrix of the mitochondrion. In prokaryotic cells, such as bacteria which lack mitochondria, the TCA reaction sequence is performed in the cytosol with the proton gradient for ATP production being across the cell’s surface (plasma membrane) rather than the inner membrane of the mitochondrion.
Products of the Citric Acid Cycle
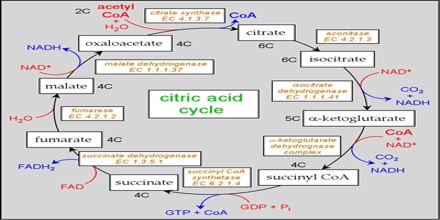
Two carbon atoms come into the citric acid cycle from each acetyl group, representing four out of the six carbons of one glucose molecule. Two carbon dioxide molecules are released on each turn of the cycle; however, these do not necessarily contain the most recently-added carbon atoms. The two acetyl carbon atoms will eventually be released on later turns of the cycle; thus, all six carbon atoms from the original glucose molecule are eventually incorporated into carbon dioxide. Each turn of the cycle forms three NADH molecules and one FADH2 molecule. These carriers will connect with the last portion of aerobic respiration to produce ATP molecules. One GTP or ATP is also made in each cycle. Several of the intermediate compounds in the citric acid cycle can be used in synthesizing non-essential amino acids; therefore, the cycle is amphibolic (both catabolic and anabolic).
Steps in the Citric Acid Cycle
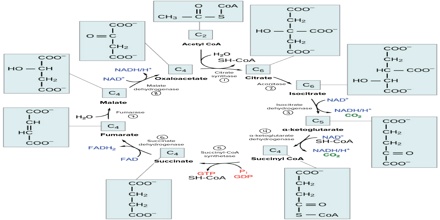
Step 1. The first step is a condensation step, combining the two-carbon acetyl group (from acetyl CoA) with a four-carbon oxaloacetate molecule to form a six-carbon molecule of citrate. CoA is bound to a sulfhydryl group (-SH) and diffuses away to eventually combine with another acetyl group. This step is irreversible because it is highly exergonic. The rate of this reaction is controlled by negative feedback and the amount of ATP available. If ATP levels increase, the rate of this reaction decreases. If ATP is in short supply, the rate increases.
Step 2. Citrate loses one water molecule and gains another as citrate is converted into its isomer, isocitrate.
Steps 3 and 4. In step three, isocitrate is oxidized, producing a five-carbon molecule, α-ketoglutarate, together with a molecule of CO2 and two electrons, which reduce NAD+ to NADH. This step is also regulated by negative feedback from ATP and NADH and by a positive effect of ADP. Steps three and four are both oxidation and decarboxylation steps, which release electrons that reduce NAD+ to NADH and release carboxyl groups that form CO2 molecules. α-Ketoglutarate is the product of step three, and a succinyl group is the product of step four. CoA binds the succinyl group to form succinyl CoA. The enzyme that catalyzes step four is regulated by feedback inhibition of ATP, succinyl CoA, and NADH.
Step 5. A phosphate group is substituted for coenzyme A, and a high-energy bond is formed. This energy is used in substrate-level phosphorylation (during the conversion of the succinyl group to succinate) to form either guanine triphosphate (GTP) or ATP. There are two forms of the enzyme, called isoenzymes, for this step, depending upon the type of animal tissue in which they are found. One form is found in tissues that use large amounts of ATP, such as heart and skeletal muscle. This form produces ATP. The second form of the enzyme is found in tissues that have a high number of anabolic pathways, such as liver. This form produces GTP. GTP is energetically equivalent to ATP; however, its use is more restricted. In particular, protein synthesis primarily uses GTP.
Step 6. Step six is a dehydration process that converts succinate into fumarate. Two hydrogen atoms are transferred to FAD, producing FADH2. The energy contained in the electrons of these atoms is insufficient to reduce NAD+ but adequate to reduce FAD. Unlike NADH, this carrier remains attached to the enzyme and transfers the electrons to the electron transport chain directly. This process is made possible by the localization of the enzyme catalyzing this step inside the inner membrane of the mitochondrion.
Step 7. In step seven, water is added to the four-carbon molecule fumarate, converting it into another four-carbon molecule called malate.
Step 8. In the last step of the citric acid cycle, oxaloacetate—the starting four-carbon compound—is regenerated by oxidation of malate. Another molecule of NAD+ start superscript, plus, end superscript is reduced to NADH in the process.
Discovery and Noble Prize

Several of the components and reactions of the citric acid cycle were established in the 1930s by the research of the Albert Szent-Györgyi, who received the Nobel Prize in Physiology or Medicine in 1937 specifically for his discoveries pertaining to fumaric acid; a key component of the cycle. The citric acid cycle itself was finally identified in 1937 by Hans Adolf Krebs and William Arthur Johnson while at the University of Sheffield, for which the former received the Nobel Prize for Physiology or Medicine in 1953.