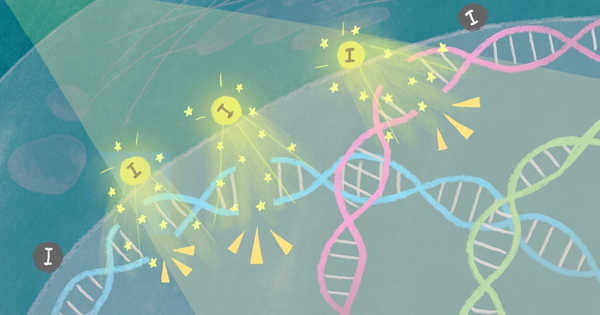
When X-rays are focused on tumor tissue containing iodine-carrying nanoparticles, cancer cell death occurs within three days. Iodine causes cell death by releasing electrons that break the DNA of the tumor. The findings were published in the journal Scientific Reports by scientists at Kyoto University’s Institute for Integrated Cell-Material Sciences (iCeMS) and colleagues from Japan and the United States.
“When a metal is exposed to light, electrons are released, a phenomenon known as the photoelectric effect. Albert Einstein’s explanation of this phenomenon in 1905 heralded the birth of quantum physics “Fuyuhiko Tamanoi, an iCeMS molecular biologist who led the study, says. “Our research provides evidence that suggests it is possible to reproduce this effect inside cancer cells.”
Cancer radiation therapy has long been ineffective at the center of tumors, where oxygen levels are low due to a lack of deep-penetrating blood vessels. When X-rays hit molecules inside the cell, they generate DNA-damaging reactive oxygen, which requires oxygen.
Researchers have found a way to enhance radiation therapy using novel iodine nanoparticles. Cancer cell death is triggered within three days when X-rays are shone onto tumor tissue containing iodine-carrying nanoparticles. The iodine releases electrons that break the tumor’s DNA, leading to cell death.
More than half of cancer patients, according to the American Cancer Society, seek radiation therapy. Radiation therapy uses a linear accelerator to direct an electron beam into a target made of a material such as tungsten, producing X-rays that doctors use to fight cancerous cells.
X-rays can deposit a large enough radiation dose to kill a tumor at high concentrations. However, even carefully aimed X-rays cause damage to surrounding tissue, so doctors face difficulties when a tumor is close to sensitive organs like the optic nerve. “They can remove the tumor, but the patient loses sight,” says Dosanjh, a trained biologist with a background in medical physics and CERN’s senior advisor for Medical Applications.
Tamanoi, Kotaro Matsumoto, and colleagues have been attempting to overcome this problem by identifying more direct ways to damage cancer DNA. Previously, they demonstrated that gadolinium-loaded nanoparticles could kill cancer cells when exposed to synchrotron-generated X-rays at 50.25 kiloelectron volts.
They created porous, iodine-carrying organosilica nanoparticles in the current study. Iodine is less expensive than gadolinium and emits electrons at lower energy levels.
The nanoparticles were dispersed by the researchers through tumor spheroids, 3D tissue containing multiple cancer cells. Irradiating the spheroids for 30 minutes with 33.2 keV X-rays resulted in their annihilation in three days. They were able to demonstrate that the optimum effect of tumor destruction occurs with 33.2 keV X-rays by systematically changing energy levels.
Further examination revealed that the nanoparticles were taken up by the tumor cells and deposited just outside their nuclei. The application of just the right amount of X-ray energy to the tissue caused iodine to release electrons, which resulted in double-strand breaks in the nuclear DNA, resulting in cell death.
“Our study is an important example of using a quantum physics phenomenon inside a cancer cell,” Matsumoto says. “A cloud of low-energy electrons appears to be generated close to DNA, causing double-strand breaks that are difficult to repair and eventually leading to programmed cell death.”
The next step is for the researchers to figure out how electrons are released from iodine atoms when they are exposed to X-rays. They are also working on putting iodine on DNA rather than near it to increase efficacy, as well as testing the nanoparticles on cancer mouse models.
The possibility of using high-energy electrons for cancer treatment, combined with new experimental dose-delivery techniques, such as zapping the tumor with a quick burst of energy via a concept known as FLASH, positions the medical field for a cancer treatment revolution, according to Dosanjh.
“With FLASH, doctors can deliver a large dose in a very short period of time,” she explains. “In this technique, cancer cells are preferentially killed while healthy cells are harmed only minimally. We have no idea how this is happening; it’s truly mind-boggling. It’s almost like magic.”