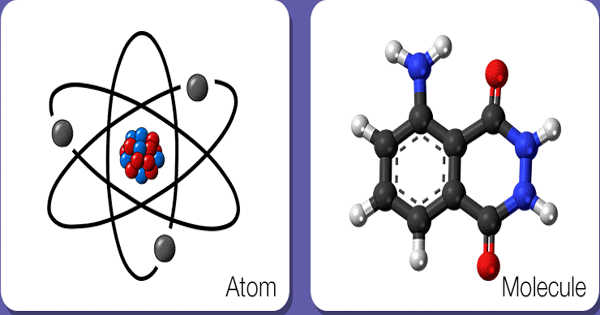
Atom is defined as the smallest unit of an element that may or may not exists independently. Molecule implies the set of atoms held together by the bond, indicating the smallest unit of a compound. So, in short, atoms are grouped together to form a molecule, which embodies the objects around us.
The whole universe is made up of the atom, and they are so small particles of an element, that we are not able to see them through bare eyes or even via microscope, but they do exist in each and every object and are affected by our activities. Atoms may or may not exist in the free state, but molecules exist in the free state.
Difference between Atom and Molecule –
ATOM
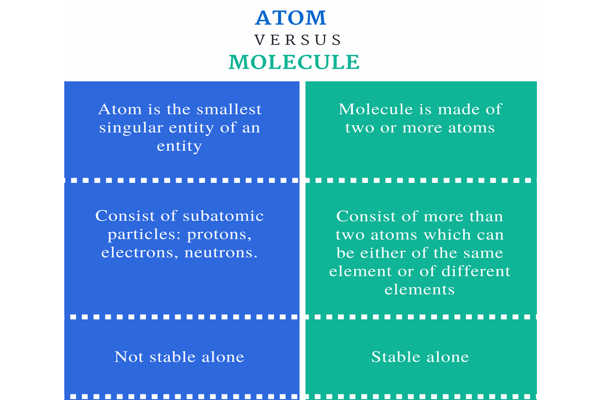
- The tiny particle of a chemical element, which may or may not exist independently is called an atom.
- Atoms comprise of the nucleus and electrons. The shape of an atom is spherical.
- The term ‘atom’ in chemistry represents the elementary unit of ordinary matter that exist in free state and contains all chemical properties. It is composed of a positively charged nucleus and surrounded by negatively charged electrons.
- Atoms are highly reactive, i.e. they participate in the chemical reaction without additional decomposition into subatomic units. However, this is not applicable to noble gas atoms.
- The nucleus consists of protons and neutrons which group together at the middle of an atom. These protons and neutrons have almost equal masses, but they vary in charge i.e. the former carry positive charge while the latter do not carry the electric charge. The positive charge in an atom is equivalent to the negative charge. Thus it is electrically neutral.
- Example: H, He, Li, O, N
MOLECULE
- Molecules refers to the set of atoms held together by bond, indicating the smallest unit of a compound. It is a small unit of matter, which exist in free state and represents the chemical properties of the substance.
- A molecule comprises of two or more, identical or different atoms, combined chemically. The molecules can be linear, angular or rectangular in shape.
- When two or more atoms are extremely close to each other, such that the electrons of the atoms can interact with one another, resulting in attraction between the atoms, called as a chemical bond.
- Molecules are less reactive, as they do not take part in the chemical reaction.
- If one or more identical atoms exist as a unit, independently, it is known as a molecule of an element, but if two or more varying elements are grouped together in a fixed proportion, by mass, to create a unit that exists freely, is termed as a molecule of a compound.
- Example: H2O, CO2, NO2, CH4