Density and mass provide physical dimensions of a given object but are completely different in application. Mass is the quantity of matter while volume is the measure of space occupied by the object. The ratio of these two aspects of the matter is known as density. While mass measures the total amount of stuff, density is how many individual particles of stuff are within a small space within the object. The measurement unit of mass is a kilogram, whereas the density is measured in kilogram per cubic meter.
Mass is a measure of how much matter there is within an object, typically given in grams. Density is the amount of mass in an object per a certain amount of volume.
Difference between Mass and Density
MASS
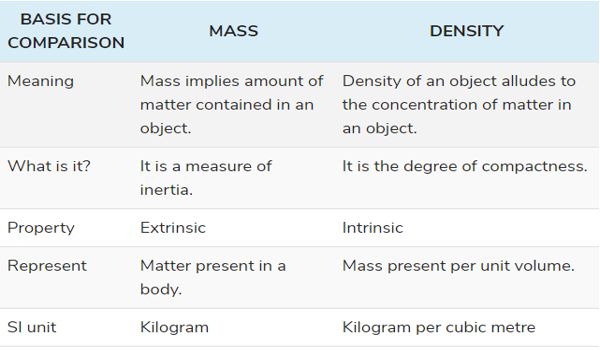
- Mass implies an amount of matter contained in an object. It is the total amount of matter present in any object.
- The term ‘mass’ can be understood as the measurement of the amount of matter present in something, i.e. how much substance is there in the object. It is measured in kilograms or its derived units.
- The important aspect of mass is that it remains constant everywhere because it is not affected by the force of gravity. For example, if a ball has a mass of 2 kg on earth then its mass on the moon will also remain the same.
- Mass is a scalar quantity, based on inertia. Inertia is the resistance of an object, to change its state of motion when the force is applied to it. The mass of an object determines the heaviness of an object without gravity, meaning that it remains constant because mass does not change with the change in place at a given moment of time.
- The mass of an object is the extrinsic property that depends on the matter present in the substance.
DENSITY
- Density of an object alludes to the concentration of matter in an object. It is the total mass present in any object with respect to its volume.
- Density is described as that amount of mass present in a substance for a given volume. It is the basic characteristic of a substance, that explains the relationship between mass and volume (space occupied).
- Density usually depicts the state of matter that is it denotes whether something is solid, liquid, or gas.
- The density of an object remains same, irrespective of its shape and size. Although, it can be changed if temperature and pressure are applied to the object, but the change in density results in the change in substance too.
- The density of an object implies the intrinsic property of an object which is not based on the quantity of matter existing in the sample.
The unit of measurement of mass is a kilogram, whereas the standard unit of density as per International Standard is kilogram per cubic metre. The SI units that represent the mass of any object are kilograms and grams whereas those of density is kg/m3 or gm/cm3.
Information Source: