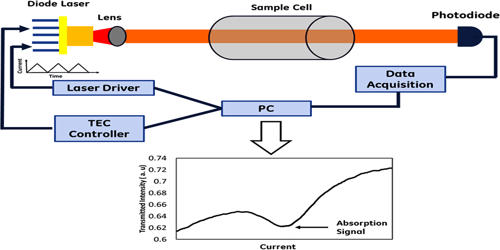
Laser absorption spectrometry (LAS) refers to techniques that use lasers to assess the concentration or amount of a species in the gas phase by absorption spectrometry (AS). There is a range of methods called laser absorption spectroscopy, where laser light is used to precisely measure the absorption features of substances. Optical spectroscopic techniques in general, and laser-based techniques in particular, have a great potential for the detection and monitoring of constituents in the gas phase. They combine a number of important properties, e.g. a high sensitivity and a high selectivity with non-intrusive and remote sensing capabilities. It is a powerful technique for qualitative and quantitative studies of atoms and molecules.
Laser absorption spectrometry has become the foremost used technique for quantitative assessments of atoms and molecules in the gas phase. A frequently used method involves that a tunable narrow-linewidth laser is tuned through some wavelength range, and the light absorption in some sample is measured as a function of that wavelength. It is also a widely used technique for a variety of other applications, e.g. within the field of optical frequency metrology or in studies of light-matter interactions. Absorption features are not always directly investigated by measuring wavelength-dependent absorption, as explained in the section on direct laser absorption spectroscopy. The most common technique is tunable diode laser absorption spectroscopy (TDLAS) which has become commercialized and is used for a variety of applications. Methods of laser absorption spectroscopy are often used for detecting the composition of materials, often including quantitative measurements of concentrations. Extensive research has been performed on the utilization of diode laser absorption spectroscopy for system monitoring and its control.
The purpose of such kinds of spectroscopy is frequently to find out details on such substances, but in other cases, one utilizes known details of substances for other purposes. For example, laser absorption spectroscopy is often used for realizing optical frequency standards, e.g. by stabilizing the wavelengths of a laser to a precisely defined absorption transition. The main features of this technique are its fast response and high sensitivity. When laser absorption spectroscopy is done on molecular gases, one can identify different species through their different absorption lines. This can be used e.g. for detecting trace gases and measuring their concentrations in the atmosphere. Its biggest disadvantage is that it relies on a measurement of a small change in power from a high level; any noise introduced by the light source or the transmission through the optical system will deteriorate the sensitivity of the technique. Loss measurements can thus be used to deduct the concentration of a gas of interest with known absorption strength in a gas mixture.