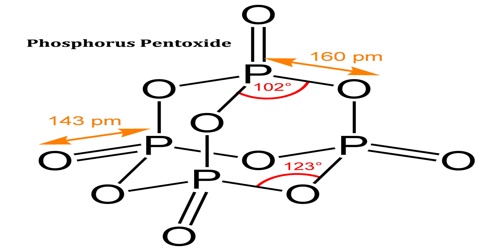
Phosphorus Pentoxide
Definition
Phosphorus pentoxide is a white odourless solid produced when phosphorus burns: has a strong affinity for water with which it forms phosphoric acids. It is a chemical compound with molecular formula. It is used in the preparation of phosphoric acids, as a drying and dehydrating agent, and in organic synthesis.

Phosphorus pentoxide is not flammable. It reacts vigorously with water and water-containing substances like wood or cotton, liberates much heat and may even cause fire. It is corrosive to metal and is very irritating – it may cause severe burns to the eye, skin, mucous membrane, and respiratory tract even at concentrations as low as 1 mg/m3.
Structure and Propertis of Phosphorus Pentoxide
This oxide is formed by combustion, in a full supply of air or oxygen, of white phosphorus (ignition temperature about 60° c.), of phosphorous oxide (50°-70° c.), of red phosphorus (about 260° c.), and of the phosphines and other combustible compounds. The white powder prepared in the laboratory or technically by these methods is always impure, containing a little phosphorous oxide, metaphosphoric acid, etc., while a part of the phosphorus usually escapes combustion and remains as red phosphorus.
It is insoluble in acetone, ammonia, soluble in sulfuric acid. It is easy to absorb moisture in the air, easy to absorb moisture, and have a strong dehydration, can even dehydrate concentrated sulfuric acid to produce sulfur trioxide, is a potent desiccant, reacts violently with water and emits heat and white smoke. Per mole of phosphorus pentoxide releases 68 kilocalories, usually generate metaphosphate with cold water, and generate mainly phosphoric acid with hot water. According to the amount of water added, it can generate different pentavalent phosphorus oxyacid, such as metaphosphoric acid, pyrophosphoric acid, orthophosphoric acid, etc. It is used to make high purity phosphoric acid, as well as gas and liquid desiccant, organic synthetic dehydrating agent.
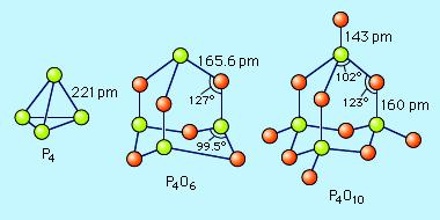
The pentoxide exists in crystalline and vitreous forms, the transformation temperature of which has been given as 440° c. Sublimation proceeds with moderate speed between 180° and 250° c. and the vapour pressure may reach 760 mm. at 360° c. When it is sublimed at 360° c. in a current of oxygen the oxide forms brilliant crystals together with some of the amorphous material which is considered to be a product of polymerisation.
It has a powerful dehydrating effect upon oxyacids and is therefore used in preparing anhydrides from these. It also removes halogen from halogen hydracids under some conditions, giving oxyhalides, e.g. POF3 from HF. Hydrogen chloride is completely absorbed by the oxide and gives a liquid from which POCl3 can be distilled.
Uses of Phosphorus Pentoxide
Phosphorus Pentoxide Used as a drying agent, dehydrating agent, sugar refining agent, and used for the preparation of phosphoric acid, phosphorous compounds and aerosol, etc. Used as semiconductor silicon doped source, dehydration drying agent, organic synthetic condensing agent and surfactant, it is also used for preparation of high purity phosphoric acid.

It may be used as one of the reaction components in the synthesis of dichlorine heptoxide (Cl2O7) and transition-metal phosphides (Ni2P, Co2P and MoP). P2O5 supported on alumina can be used for the solvent-free and microwave-assisted preparation of 1, 5-benzodiazepine analogs. P2O5/KX (X = Br, I) reagent system may be used for the transformation of alcohols into the corresponding alkyl iodides and bromides.
It is used as raw material for the production of high purity phosphoric acid, phosphates and phosphate, also used for the preparation of phosphorus pentoxide sol and H-based aerosol. It can be used as a desiccant of gases and liquids, dehydrating agent for organic synthesis, antistatic agents of synthetic fiber and sugar refining agent. It is also used in the manufacture of optical glass, UV transparent glass, insulating glass, microcrystalline glass and opaque glass, etc, so as to improve the dispersion coefficient and UV light through ability of the glass. Also used in the production of pharmaceuticals, pesticides, surfactants.
Reference: chemicalbook.com, atomistry.com, dictionary.com, wikipedia.