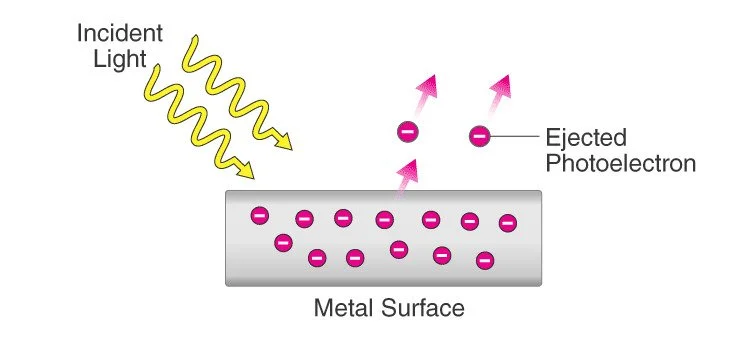
The photoelectric effect is a phenomenon in which electrons are emitted from a material surface when it is exposed to light or other electromagnetic radiation. The effect was first observed and explained by Albert Einstein in 1905, and it played a crucial role in the development of quantum mechanics.
When electromagnetic radiation, such as light, strikes a material, electrons are emitted. Photoelectrons are electrons that are emitted in this manner. The phenomenon is studied in condensed matter physics, solid state and quantum chemistry to draw conclusions about the properties of atoms, molecules, and solids. The effect has found application in electronic devices specialized in light detection and precisely timed electron emission.
The photoelectric effect occurs when a photon, which is a particle of light, interacts with an electron in the material. If the photon has enough energy, it can transfer that energy to the electron and knock it out of the material. The electron that is emitted is called a photoelectron, and its energy is equal to the energy of the photon minus the energy required to overcome the binding energy of the electron to the material.
The experimental results contradict classical electromagnetism, which predicts that continuous light waves transfer energy to electrons, which are then emitted when they accumulate enough energy. A change in light intensity would theoretically change the kinetic energy of the emitted electrons, with sufficiently dim light resulting in delayed emission.
The experimental results, on the other hand, show that electrons are dislodged only when the light frequency exceeds a certain threshold, regardless of the intensity or duration of exposure. Because a high-intensity low-frequency beam does not accumulate the energy required to produce photoelectrons, as would be the case if light’s energy accumulated over time from a continuous wave, Albert Einstein proposed that a beam of light is a swarm of discrete energy packets known as photons, rather than a wave propagating through space.
History
Wilhelm Ludwig Franz Hallwachs first proposed the photoelectric effect in 1887, and Heinrich Rudolf Hertz performed the experimental verification. When a surface is exposed to electromagnetic radiation at a higher threshold frequency, the radiation is absorbed and electrons are emitted, they discovered. Today, the photoelectric effect is studied as a phenomenon in which a material absorbs electromagnetic radiation and emits electrically charged particles.
Application
The photoelectric effect has many practical applications, including in photovoltaic cells used for solar energy, in photoelectric sensors used in digital cameras, and in electron microscopes used to image materials at the atomic scale. It also played a key role in the development of quantum mechanics, which revolutionized our understanding of the behavior of matter and energy at the atomic and subatomic level.