Thermodynamics is a branch of physics; it deals with the transfer of energy from one place to another and from one form to another. The key concept is that heat is a form of energy corresponding to a definite amount of mechanical work. William Thomson coined the term thermodynamics in 1749. It is derived from two Greek words “thermes” meaning heat, and “dynamikos” meaning powerful. American biophysicist Donald Haynie claims that thermodynamics was coined in 1840 from the Greek root θέρμη therme, meaning “heat”, and δύναμις dynamis, meaning “power”. By 1858, thermo-dynamics, as a functional term, was used in William Thomson’s paper “An Account of Carnot’s Theory of the Motive Power of Heat.”
We can define thermodynamics as:
“The branch of Physics that deals with heat and temperature, and their relation to energy, work, radiation, and properties of matter.”
To be specific, it explains how thermal energy is converted to or from other forms of energy and how matter is affected by this process. Thermal energy is the energy that comes from heat. This heat is generated by the movement of tiny particles within an object. The faster these particles move, the more heat is generated.
Heat was not formally recognized as a form of energy until about 1798, when Count Rumford (Sir Benjamin Thompson), a British military engineer, noticed that limitless amounts of heat could be generated in the boring of cannon barrels and that the amount of heat generated is proportional to the work done in turning a blunt boring tool. Rumford’s observation of the proportionality between heat generated and work done lies at the foundation of thermodynamics. Another pioneer was the French military engineer Sadi Carnot, who introduced the concept of the heat-engine cycle and the principle of reversibility in 1824. Carnot’s work concerned the limitations on the maximum amount of work that can be obtained from a steam engine operating with a high-temperature heat transfer as its driving force. Later that century, these ideas were developed by Rudolf Clausius, a German mathematician and physicist, into the first and second laws of thermodynamics, respectively.
The initial application of thermodynamics to mechanical heat engines was quickly extended to the study of chemical compounds and chemical reactions. Chemical thermodynamics studies the nature of the role of entropy in the process of chemical reactions and has provided the bulk of expansion and knowledge of the field. Other formulations of thermodynamics emerged. Statistical thermodynamics, or statistical mechanics, concerns itself with statistical predictions of the collective motion of particles from their microscopic behavior. In 1909, Constantin Carathéodory presented a purely mathematical approach in an axiomatic formulation, a description often referred to as geometrical thermodynamics.
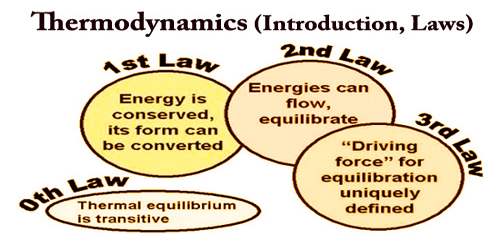
Laws of Thermodynamics –
The laws of thermodynamics define the fundamental physical quantities like energy, temperature and entropy that characterize thermodynamic systems at thermal equilibrium. The laws represent how these quantities behave under various circumstances.
There are four laws of thermodynamics and are given below:
Zeroth law of thermodynamics – The zeroth law of thermodynamics states: If two systems are each in thermal equilibrium with a third, they are also in thermal equilibrium with each other.
This statement implies that thermal equilibrium is an equivalence relation on the set of thermodynamic systems under consideration. Systems are said to be in equilibrium if the small, random exchanges between them (e.g. Brownian motion) do not lead to a net change in energy. This law is tacitly assumed in every measurement of temperature. Thus, if one seeks to decide whether two bodies are at the same temperature, it is not necessary to bring them into contact and measure any changes of their observable properties in time. The law provides an empirical definition of temperature, and justification for the construction of practical thermometers.
The zeroth law was not initially recognized as a separate law of thermodynamics, as its basis in thermodynamical equilibrium was implied in the other laws. The first, second, and third laws had been explicitly stated already, and found common acceptance in the physics community before the importance of the zeroth law for the definition of temperature was realized. As it was impractical to renumber the other laws, it was named the zeroth law.
First Law of Thermodynamics – The first law of thermodynamics or the law of conservation of energy states that: Energy can neither be created nor destroyed, but it can be changed from one form to another.
The first law is put into action by considering the flow of energy across the boundary separating a system from its surroundings. Consider the classic example of a gas enclosed in a cylinder with a movable piston. The walls of the cylinder act as the boundary separating the gas inside from the world outside and the movable piston provides a mechanism for the gas to do work by expanding against the force holding the piston (assumed frictionless) in place. If the gas does work W as it expands, and/or absorbs heat Q from its surroundings through the walls of the cylinder, then this corresponds to a net flow of energy W − Q across the boundary to the surroundings. In order to conserve the total energy U, there must be a counterbalancing change ΔU = Q − W (1) in the internal energy of the gas. The first law provides a kind of strict energy accounting system in which the change in the energy account (ΔU) equals the difference between deposits (Q) and withdrawals (W).
Adapted for thermodynamics, this law is an expression of the principle of conservation of energy, which states that energy can be transformed (changed from one form to another), but cannot be created or destroyed. Internal energy is a principal property of the thermodynamic state, while heat and work are modes of energy transfer by which a process may change this state. A change of internal energy of a system may be achieved by any combination of heat added or removed and work performed on or by the system. As a function of the state, the internal energy does not depend on the manner, or on the path through intermediate steps, by which the system arrived at its state.
Second Law of Thermodynamics – The second law of thermodynamics states that:
‘Energy in the form of heat only flows from regions of higher temperature to that of lower temperature.’
Many individuals take this statement lightly and for granted, but it has an extensive impact and consequence. This is why it costs money to run an air conditioner. The human body obeys the second law of thermodynamics too.
The second law is an observation of the fact that over time, differences in temperature, pressure, and chemical potential tend to even out in a physical system that is isolated from the outside world. Entropy is a measure of how much this process has progressed. The entropy of an isolated system which is not in equilibrium will tend to increase over time, approaching a maximum value at equilibrium. However, principles guiding systems that are far from equilibrium are still debatable. One of such principle is the maximum entropy production principle. It states that non-equilibrium systems behave in such a way as to maximize its entropy production.
In classical thermodynamics, the second law is a basic postulate applicable to any system involving heat energy transfer; in statistical thermodynamics, the second law is a consequence of the assumed randomness of molecular chaos. There are many versions of the second law, but they all have the same effect, which is to express the phenomenon of irreversibility in nature.
Third Law of Thermodynamics – The Third Law states that the entropy of a pure crystal at absolute zero is zero. As explained above, entropy is sometimes called “waste energy,” i.e., an energy that is unable to do work, and since there is no heat energy whatsoever at absolute zero, there can be no waste energy. Entropy is also a measure of the disorder in a system, and while a perfect crystal is by definition perfectly ordered, any positive value of temperature means there is motion within the crystal, which causes the disorder. For these reasons, there can be no physical system with lower entropy, so entropy always has a positive value.
Alternate definitions include “the entropy of all systems and of all states of a system is smallest at absolute zero,” or equivalently “it is impossible to reach the absolute zero of temperature by any finite number of processes”. Absolute zero, at which all activity would stop if it were possible to achieve, is −273.15 °C (degrees Celsius), or −459.67 °F (degrees Fahrenheit), or 0 K (kelvin), or 0° R (degrees Rankine).
The science of thermodynamics has been developed over centuries, and its principles apply to nearly every device ever invented. Its importance in modern technology cannot be overstated.
Information Sources: